




















中国农业科技导报 ›› 2024, Vol. 26 ›› Issue (8): 140-150.DOI: 10.13304/j.nykjdb.2023.0238
• 动植物健康 • 上一篇
张俊蕾1,2( ), 盖晓彤2, 赵正婷3, 刘弟4, 王金凤1, 姜宁2(
), 盖晓彤2, 赵正婷3, 刘弟4, 王金凤1, 姜宁2( ), 刘雅婷1,4(
), 刘雅婷1,4( )
)
收稿日期:2023-03-29
接受日期:2023-05-24
出版日期:2024-08-15
发布日期:2024-08-12
通讯作者:
姜宁,刘雅婷
作者简介:张俊蕾 E-mail:jleiZHANGg@163.com
基金资助:
Junlei ZHANG1,2( ), Xiaotong GE2, Zhengting ZHAO3, Di LIU4, Jinfeng WANG1, Ning JIANG2(
), Xiaotong GE2, Zhengting ZHAO3, Di LIU4, Jinfeng WANG1, Ning JIANG2( ), Yating LIU1,4(
), Yating LIU1,4( )
)
Received:2023-03-29
Accepted:2023-05-24
Online:2024-08-15
Published:2024-08-12
Contact:
Ning JIANG,Yating LIU
摘要:
为快速检测烟草番茄斑萎病毒(tomato spotted wilt virus,TSWV),基于TSWV核外壳蛋白(nucleocapsid protein,NP)保守序列设计5组引物进行筛选,采用单一变量法对反应温度、时间及反应体系中dNTPs、Mg2+、甜菜碱的含量和内外引物比等参数进行优化,建立烟草TSWV反转录环介导等温扩增(loop-mediated isothermal amplification,RT-LAMP)检测体系,并采用RT-PCR(revers transcription-polymerase chain reaction)检测方法进行平行比对试验,验证优化后RT-LAMP的特异性、灵敏性及实用性。结果表明,烟草TSWV RT-LAMP检测体系最佳引物组为TS-N-4,在25 μL的反应体系中各组分最佳加入量为缓冲液2.5 μL、100 mmol·L-1 MgSO4 0.5 μL、10 mmol·L-1 dNTPs 0.5 μL、10 mmol·L-1 FIP/BIP 1.5 μL、10 mmol·L-1 F3/B3 0.5 μL、10 mmol·L-1 LF/LB 1.5 μL、5 mmol·L-1 Betaine 6 μL、Bst 2.0 WarmStar DNA Polymerase(8 000 U·mL-1) 0.5 μL、M-MLV 酶(10 000 U·mL-1) 0.125 μL、RNA (≥64.7 fg)1 μL,DEPC H2O补至25 μL;最佳反应温度和时间分别为58 ℃、60 min。优化后的RT-LAMP灵敏度是RT-PCR的1 000倍,且田间样品检测结果与RT-PCR相符。建立的RT-LAMP方法特异性强、灵敏度高、操作简单,对于烟草TSWV的检测及监控有重要意义。
中图分类号:
张俊蕾, 盖晓彤, 赵正婷, 刘弟, 王金凤, 姜宁, 刘雅婷. 烟草番茄斑萎病毒RT-LAMP检测体系的建立及优化[J]. 中国农业科技导报, 2024, 26(8): 140-150.
Junlei ZHANG, Xiaotong GE, Zhengting ZHAO, Di LIU, Jinfeng WANG, Ning JIANG, Yating LIU. Establishment and Optimization of RT-LAMP Assay System for Tobacco Tomato Spotted Wilt Virus[J]. Journal of Agricultural Science and Technology, 2024, 26(8): 140-150.
引物 Primer | 序列 Sequence(5’-3’) | |
|---|---|---|
| TS-N-1 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | AGCTTTGAGATGATCAGTGT | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATAC | |
| BIP | TAAGGCTTCCCTGGTGTCAT-TGTCTTGGCTATATATCAGGATG | |
| TS-N-2 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | AGCTTTGAGATGATCAGTGT | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATA | |
| BIP | TAAGGCTTCCCTGGTGTCATA-TGTCTTGGCTATATATCAGGATG | |
| TS-N-3 | F3 | TCTTCACCTGATCTTCATTCA |
| B3 | TCTGTGAGGCTTGCCATA | |
| FIP | CGGGATCGATCCAAAGAAGTATGAC-CTTTTTAGCACAGTGCAAAC | |
| BIP | GCATCCTGATATATAGCCAAGACAA-TGCTGGGAGGTAGCTTAC | |
| TS-N-4 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | AGCTTTGAGATGATCAGTGT | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATA | |
| BIP | TAAGGCTTCCCTGGTGTCAT-TGTCTTGGCTATATATCAGGATG | |
| LF | GATCAGGTGAAGAAAGGGAAAGAG | |
| LB | ACTTCTTTGGATCGATCCCGA | |
| TS-N-5 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | GATAGCTTTGAGATGATCAGTG | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATA | |
| BIP | TAAGGCTTCCCTGGTGTCAT-TTGTCTTGGCTATATATCAGGATG | |
| RT-PCR | TSWV-F | CTGCACAATCCCAAGACA |
| TSWV-R | GCTAAGAGATTGAGRAATGGTATAATAGATTC | |
表 1 RT-LAMP和RT-PCR特异性检测TSWV引物
Table 1 Specific primers for RT-LAMP and RT-PCR for TSWV
引物 Primer | 序列 Sequence(5’-3’) | |
|---|---|---|
| TS-N-1 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | AGCTTTGAGATGATCAGTGT | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATAC | |
| BIP | TAAGGCTTCCCTGGTGTCAT-TGTCTTGGCTATATATCAGGATG | |
| TS-N-2 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | AGCTTTGAGATGATCAGTGT | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATA | |
| BIP | TAAGGCTTCCCTGGTGTCATA-TGTCTTGGCTATATATCAGGATG | |
| TS-N-3 | F3 | TCTTCACCTGATCTTCATTCA |
| B3 | TCTGTGAGGCTTGCCATA | |
| FIP | CGGGATCGATCCAAAGAAGTATGAC-CTTTTTAGCACAGTGCAAAC | |
| BIP | GCATCCTGATATATAGCCAAGACAA-TGCTGGGAGGTAGCTTAC | |
| TS-N-4 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | AGCTTTGAGATGATCAGTGT | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATA | |
| BIP | TAAGGCTTCCCTGGTGTCAT-TGTCTTGGCTATATATCAGGATG | |
| LF | GATCAGGTGAAGAAAGGGAAAGAG | |
| LB | ACTTCTTTGGATCGATCCCGA | |
| TS-N-5 | F3 | ACTTCCTTTAGCATTAGGATTG |
| B3 | GATAGCTTTGAGATGATCAGTG | |
| FIP | GTGCTAAAAAGCAAAGCATTTGAAA-GGAGCTAAGTATAGCAGCATA | |
| BIP | TAAGGCTTCCCTGGTGTCAT-TTGTCTTGGCTATATATCAGGATG | |
| RT-PCR | TSWV-F | CTGCACAATCCCAAGACA |
| TSWV-R | GCTAAGAGATTGAGRAATGGTATAATAGATTC | |
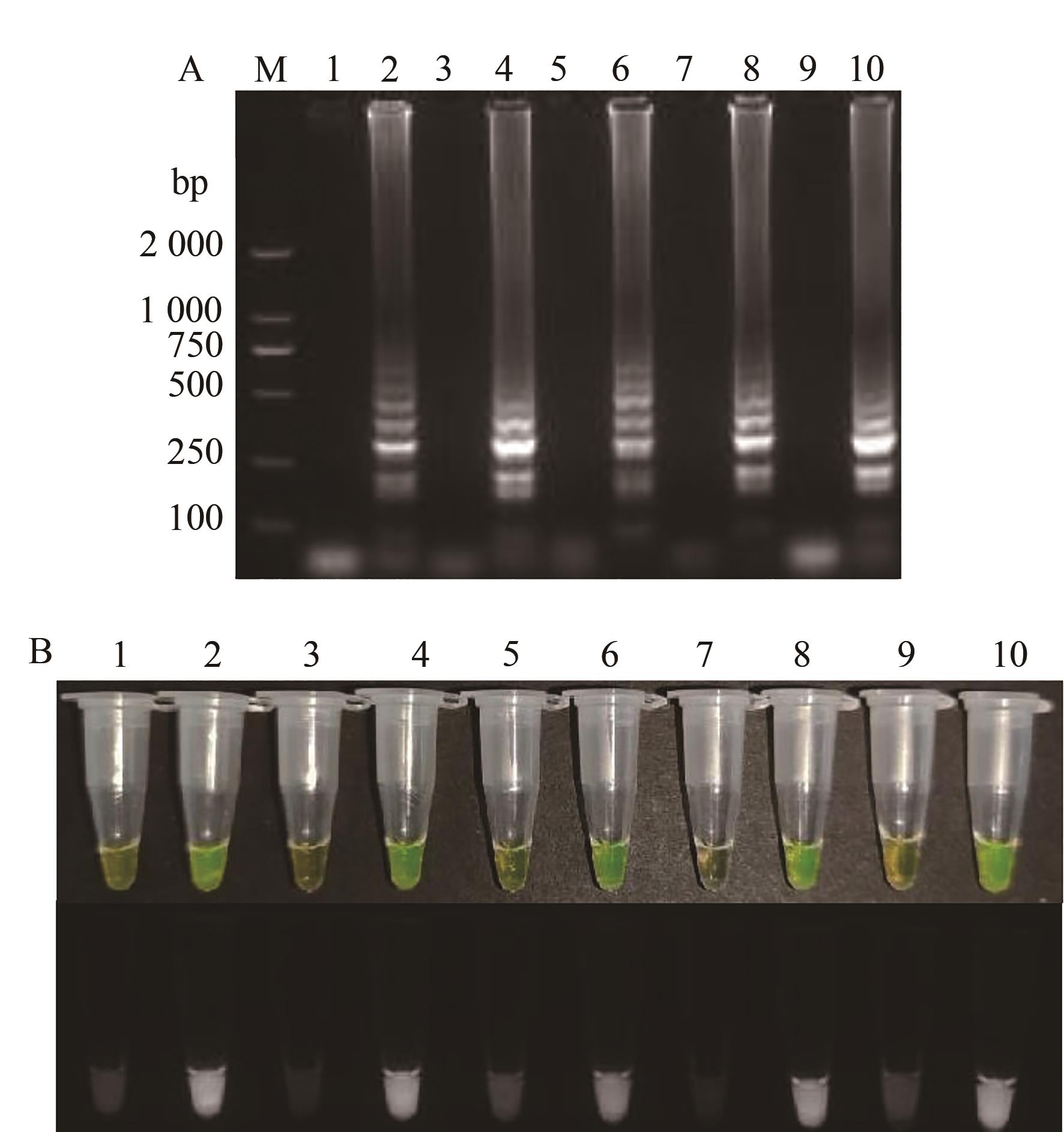
图1 TSWV不同RT-LAMP引物扩增结果A:凝胶电泳图;B:荧光可视化结果;M—2 000 DNA marker;1、3、5、7、9—阴性对照;2、4、6、8、10—TSWV的RT-LAMP引物TS-N-1~TS-N-5
Fig. 1 Amplification results of TSWV with different RT-LAMP primersA: Gel electrophoresis; B: Fluorescence visualization results; M—2 000 DNA marker; 1, 3, 5, 7, 9—Negative control; 2, 4, 6, 8, 10—RT-LAMP primers TS-N-1~TS-N-5 of TSWV
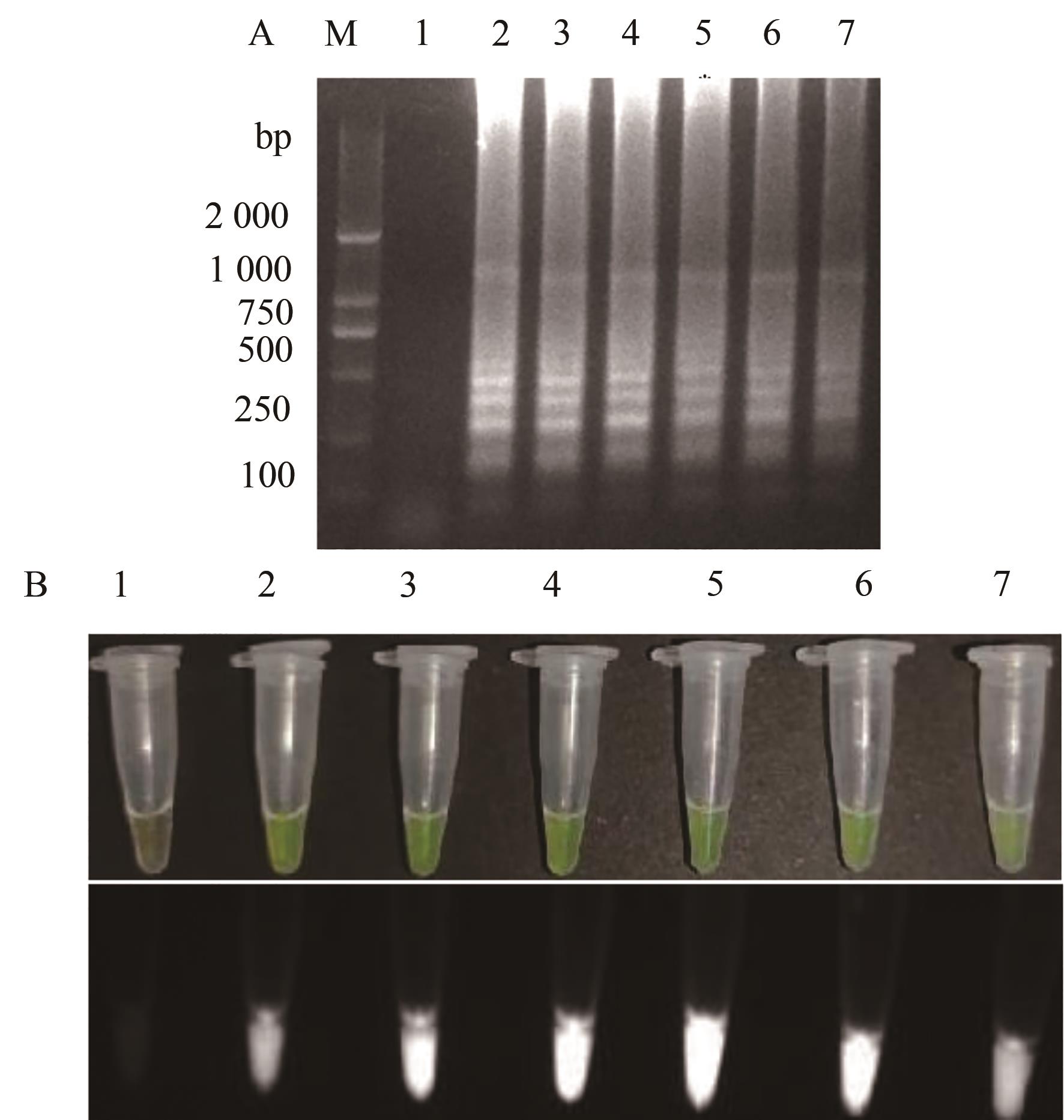
图2 TSWV RT-LAMP反应温度优化A:凝胶电泳;B:荧光可视化结果;M—2 000 DNA marker;1—阴性对照;2~7—6个反应温度58、60、62、64、66、68 ℃
Fig. 2 TSWV RT-LAMP reaction temperature optimizationA: Gel electrophoresis; B: Fluorescence visualization results: M—2 000 DNA marker; 1—Negative control; 2~7—6 reaction time gradients 58, 60, 62, 64, 66, 68℃
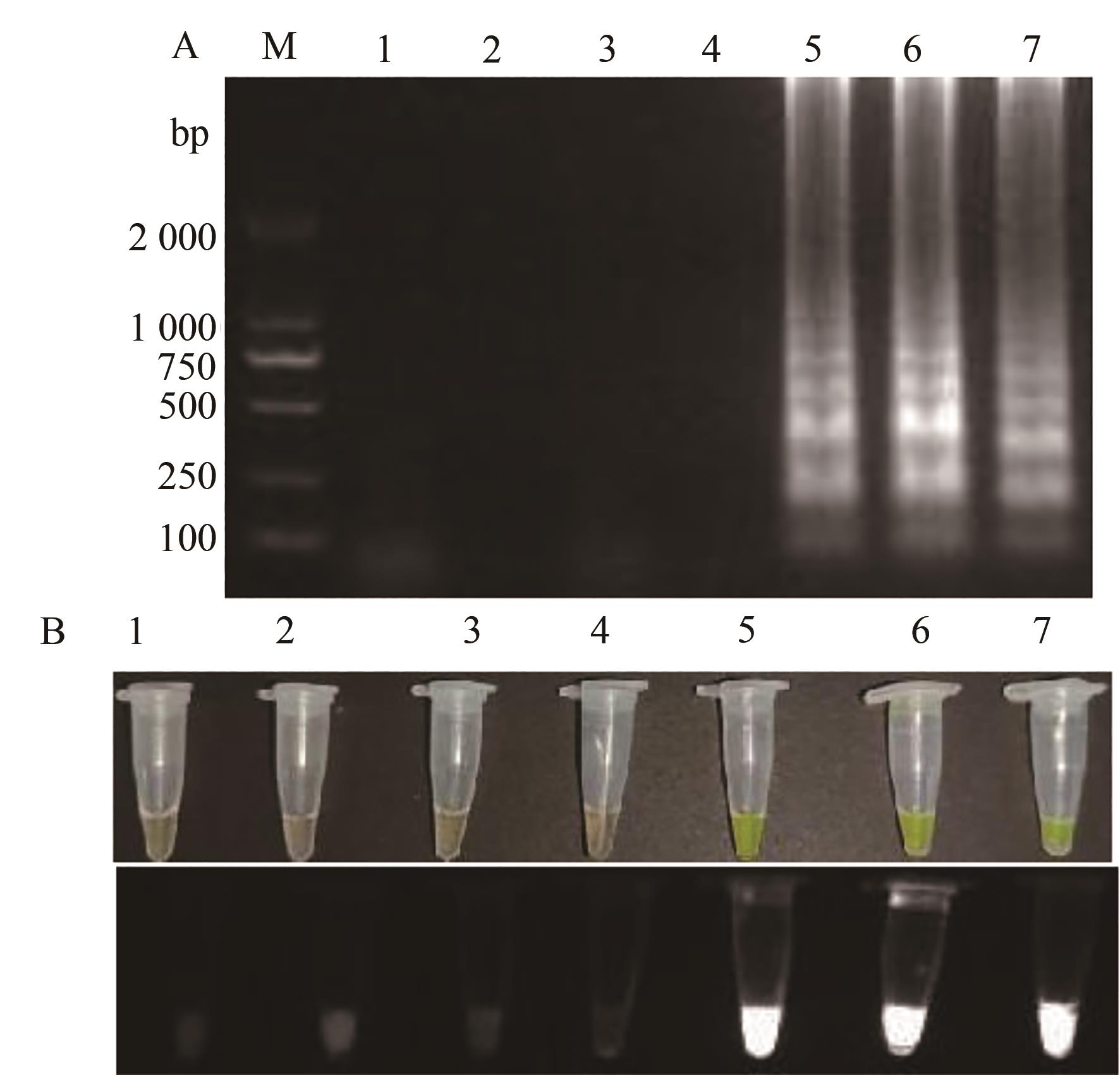
图3 TSWV RT-LAMP反应时间的优化A:凝胶电泳;B:荧光可视化结果;M—2 000 DNA marker;1—阴性对照;2~7—6个反应温时间30、40、50、60、70、80 min
Fig. 3 Optimization of the TSWV RT-LAMP reaction timesA: Gel electrophoresis; B: Fluorescence visualization results: M—2 000 DNA marker; 1—Negative control; 2~7—6 reaction time gradient 30,40,50,60,70,80 min
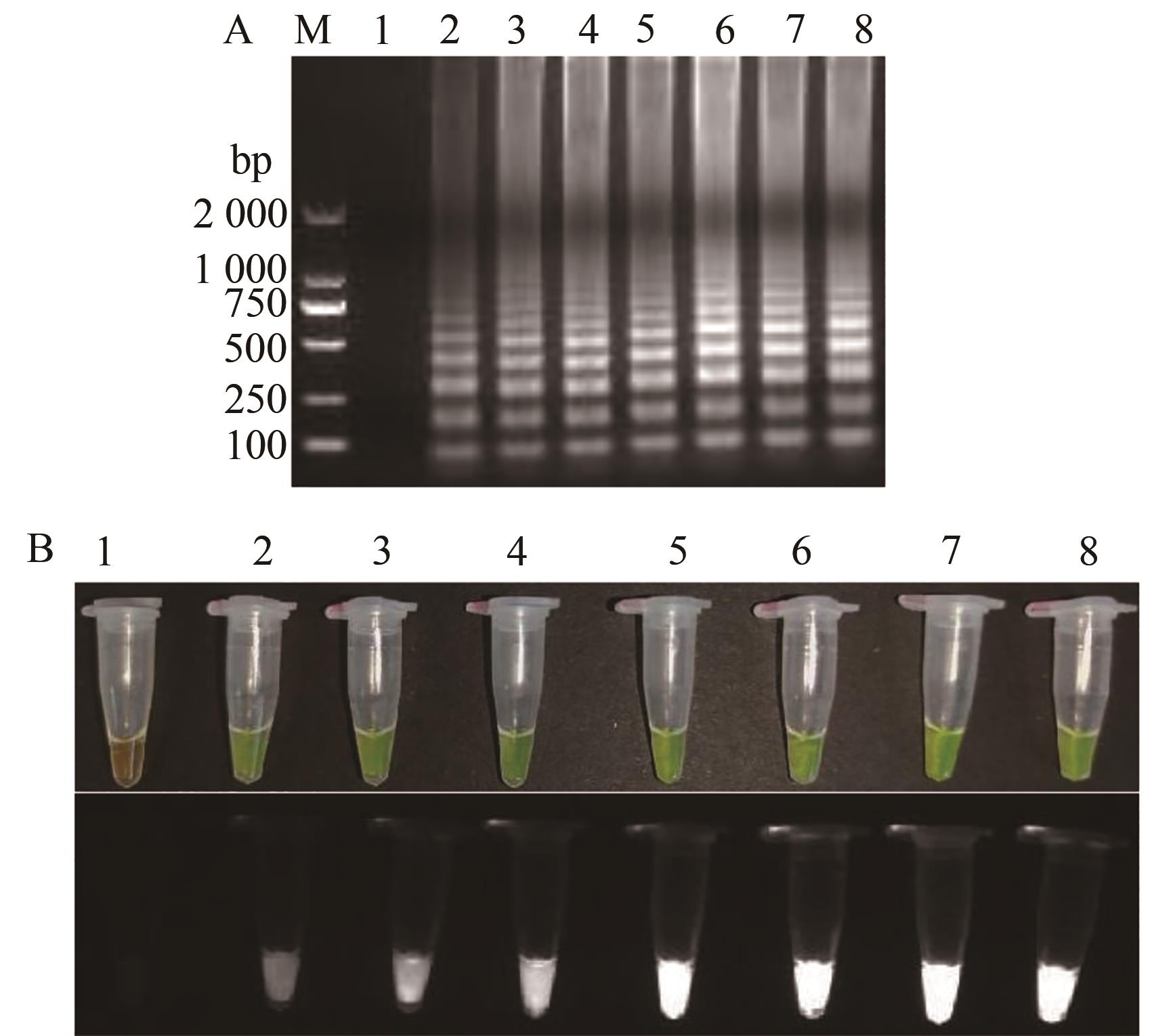
图4 TSWV RT-LAMP甜菜碱含量的优化A:凝胶电泳;B:荧光可视化结果;M—2 000 DNA marker;1—阴性对照;2~8—7个反应含量0.0、0.6、0.8、1.0、1.2、1.4、1.6 mmol·L-1
Fig. 4 Optimization of betaine content of TSWV RT-LAMPA: Gel electrophoresis; B: Fluorescence visualization results; M—2 000 DNA marker; 1—Negative control; 2~8—7 reaction contents 0.0, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6 mmol·L-1
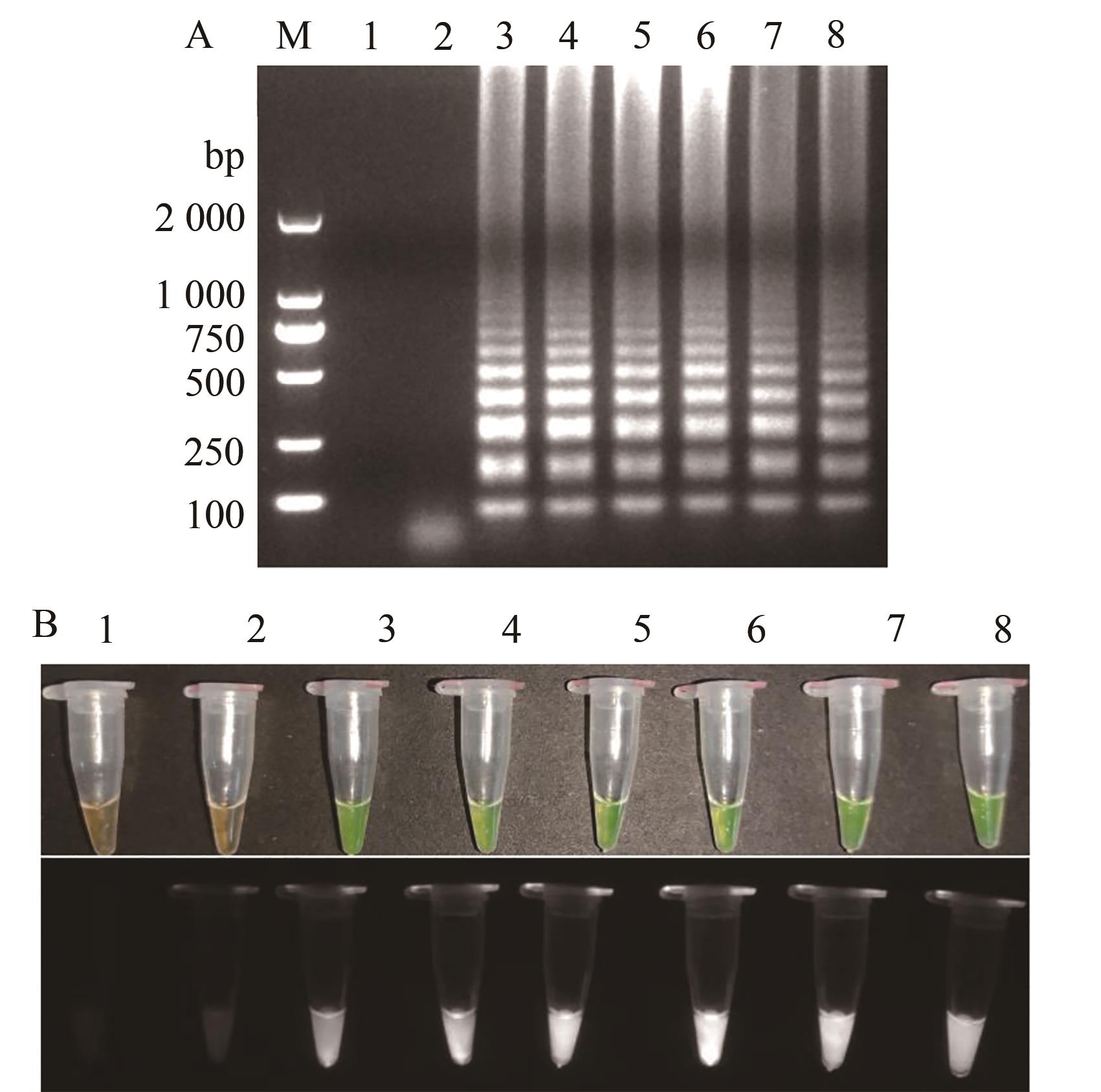
图5 TSWV RT-LAMP dNTPs反应含量的优化A:凝胶电泳;B:荧光可视化结果;M—2 000 DNA marker;1—阴性对照;2~8—7个反应含量0.0、0.2、0.6、1.0、1.4、1.8、2.2 mmol·L-1
Fig. 5 Optimization of TSWV RT-LAMP dNTPs reaction contentA: Gel electrophoresis; B: Fluorescence visualization results; M—2 000 DNA marker; 1—Negative control; 2~8—7 reaction contents 0.0, 0.2, 0.6, 1.0, 1.4, 1.8, 2.2 mmol·L-1
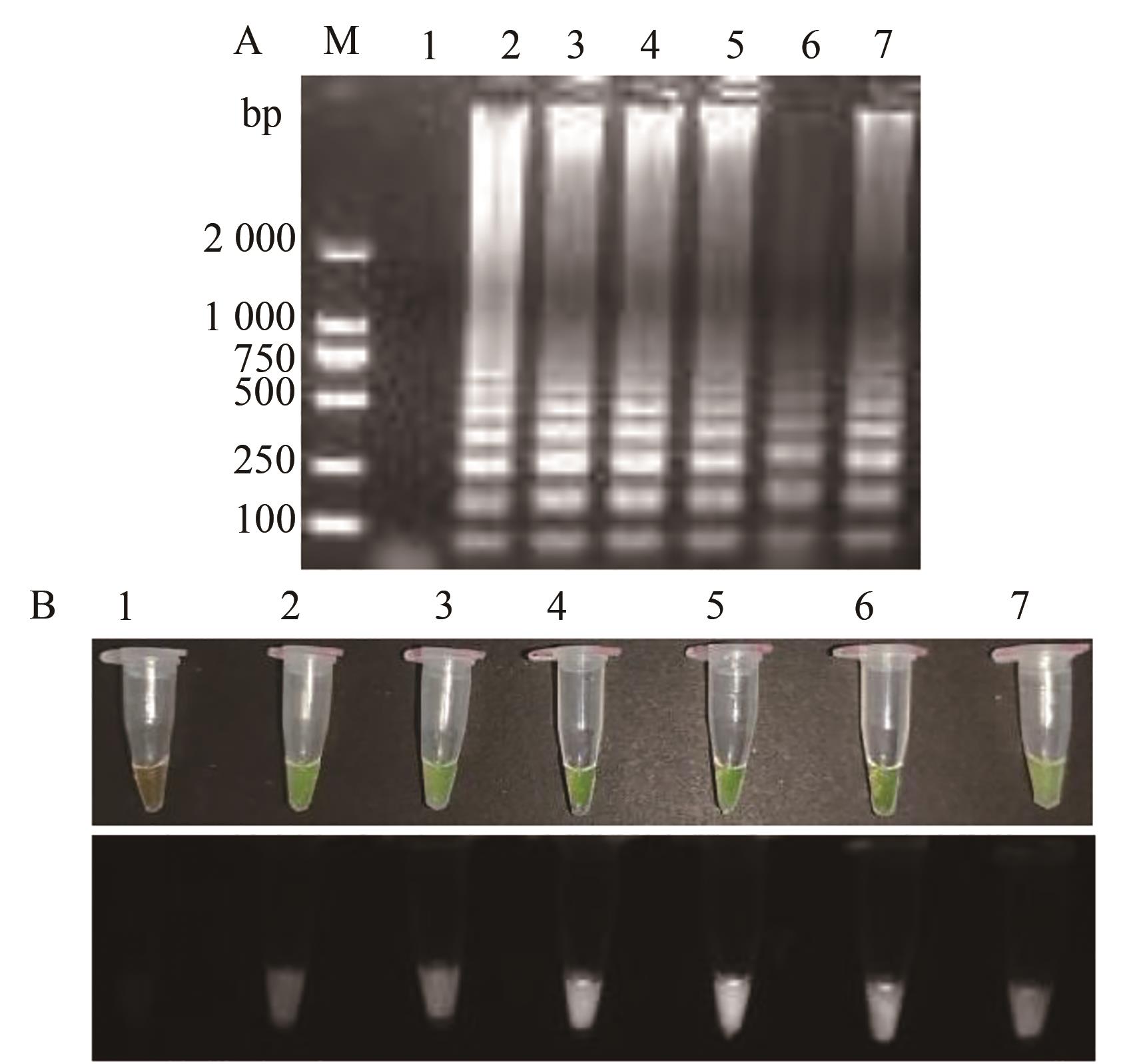
图6 TSWV RT-LAMP Mg2+反应含量的优化A:凝胶电泳;B:荧光可视化结果;M—2 000 DNA marker;1—阴性对照;2~7—6个反应含量2、4、6、8、10、12 mmol·L-1
Fig. 6 Optimization of TSWV RT-LAMP Mg2+ reaction contentA: Gel electrophoresis; B: Fluorescence visualization results; M—2 000 DNA marker; 1—Negative control; 2~7—6 reaction contents 2, 4, 6, 8, 10, 12 mmol·L-1
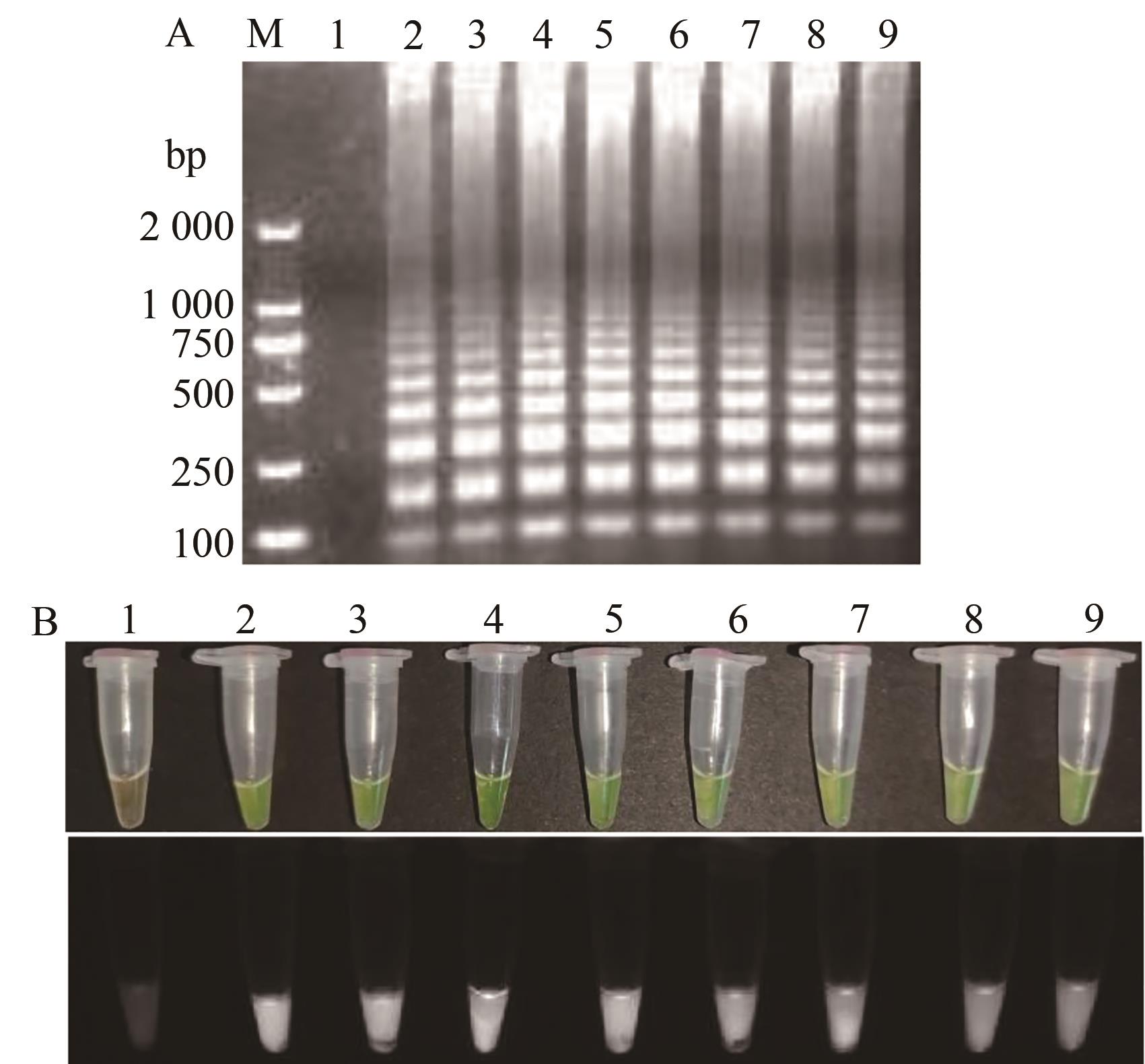
图7 TSWV RT-LAMP内外引物用量比的优化A:凝胶电泳;B:荧光可视化结果;M—2 000 DNA marker;1—阴性对照;2~9—8个引物用量比梯度1∶1~8∶1
Fig. 7 Optimization of inside and outside primers content ratio of TSWV RT-LAMPA: Gel electrophoresis; B: Fluorescence visualization results;M—2 000 DNA marker; 1—Negative control; 2~9—8 primer ratio gradient 1∶1~8∶1
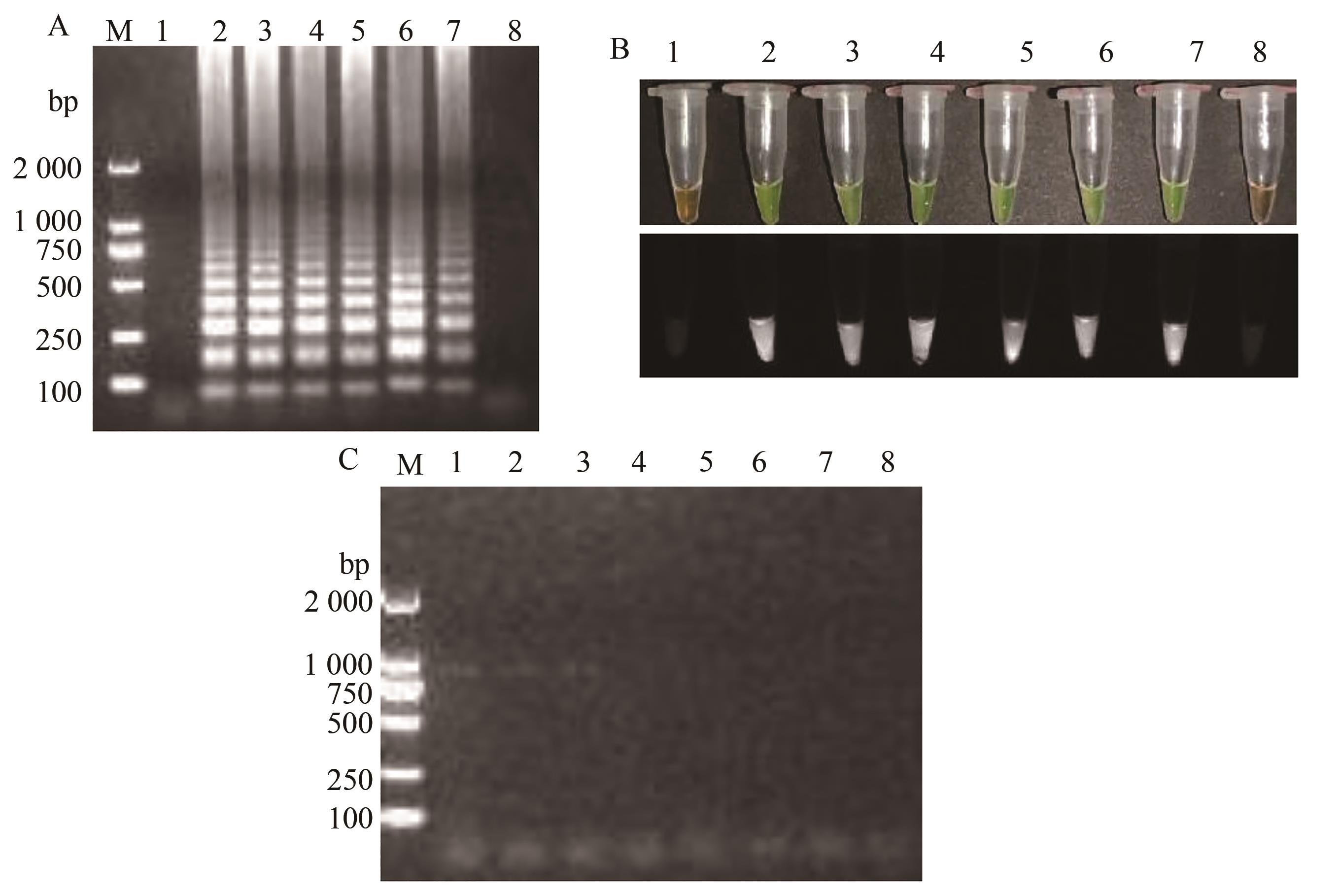
图8 TSWV RT-LAMP灵敏度检测A:RT-LAMP凝胶电泳;1—阴性对照;2~8—7个RNA的含量642.7×100 ~ 642.7×10-6 ng·μL-1;B:RT-PCR凝胶电泳图;1~7—7个RNA的含量642.7×100~642.7×10-6 ng·μL-1;8—阴性对照;M—2 000 DNA marker;C:荧光可视化;1—阴性对照;2~8—7个RNA的含量642.7×100~642.7×10-6 ng·μL-1
Fig. 8 TSWV RT-LAMP sensitivity detectionA: Gel electrophoresis diagram; 1—Negative control; 2~8—Seven RNA reaction contents of 642.7×100~642.7×10-6 ng·μL-1;B: Gel electrophoresis diagram; 1~7—Seven RNA reaction contents of 642.7×100~642.7×10-6 ng·μL-1;8—Negative control;M—2 000 DNA marker; C: Fluorescence visualization results; 1—Negative control; 2~8—Seven RNA reaction contents of 642.7×100~642.7×10-6 ng·μL-1
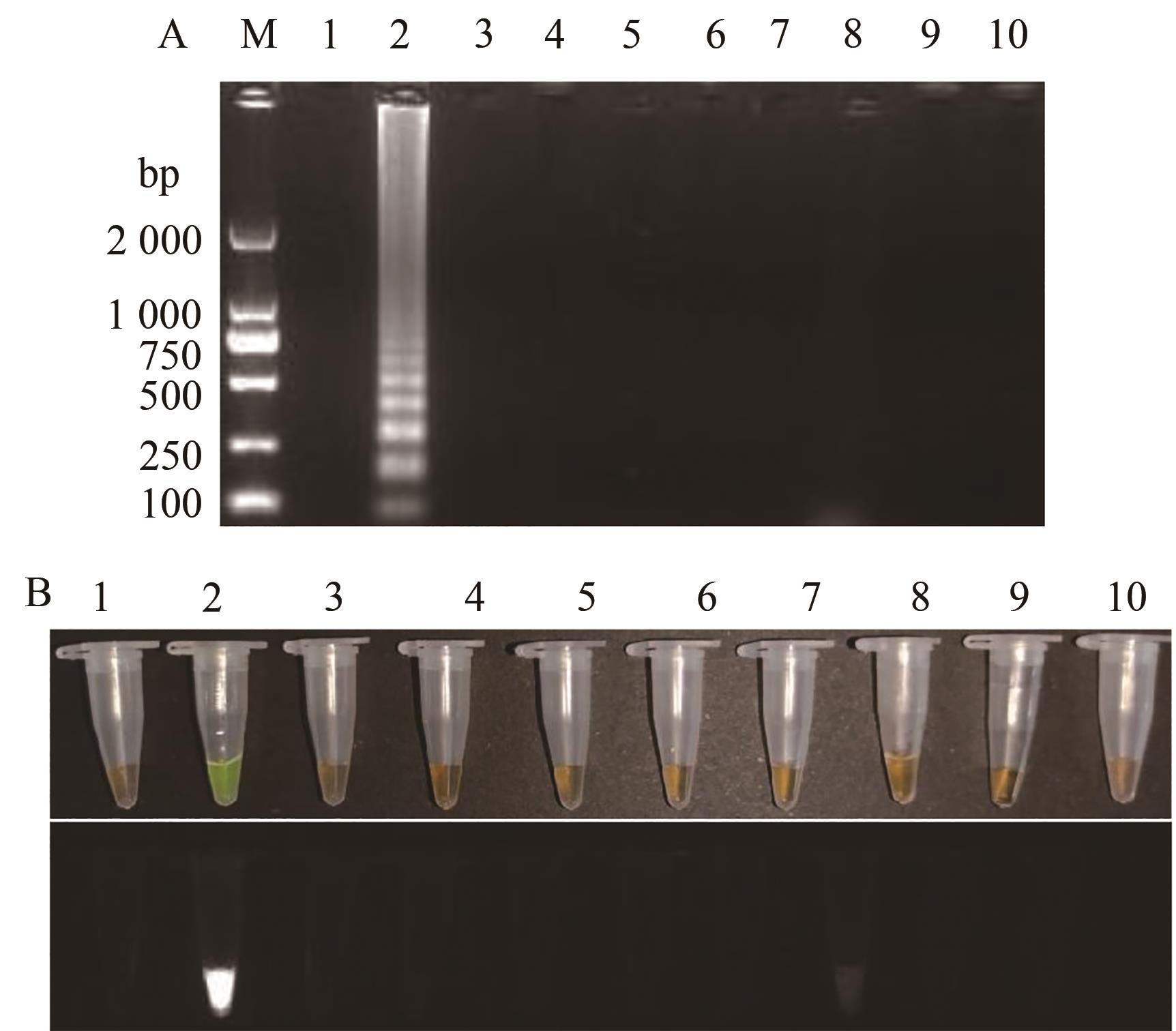
图9 TSWV RT-LAMP特异性检测A:凝胶电泳图;B:荧光可视化结果;M—2000 DNA marker;1—阴性对照;2~10—TSWV、TMV、CMV、ToBRFV、HCRV、TZSV、TVBMV、ChiVMV、PVY的RNA
Fig. 9 TSWV RT-LAMP specificity detectionA: Gel electrophoresis; B: Fluorescence visualization results; M—2 000 DNA marker; 1—Negative control; 2~10—RNA for TSWV, TMV, CMV, ToBRFV, HCRV, TZSV, TVBMV, ChiVMV and PVY
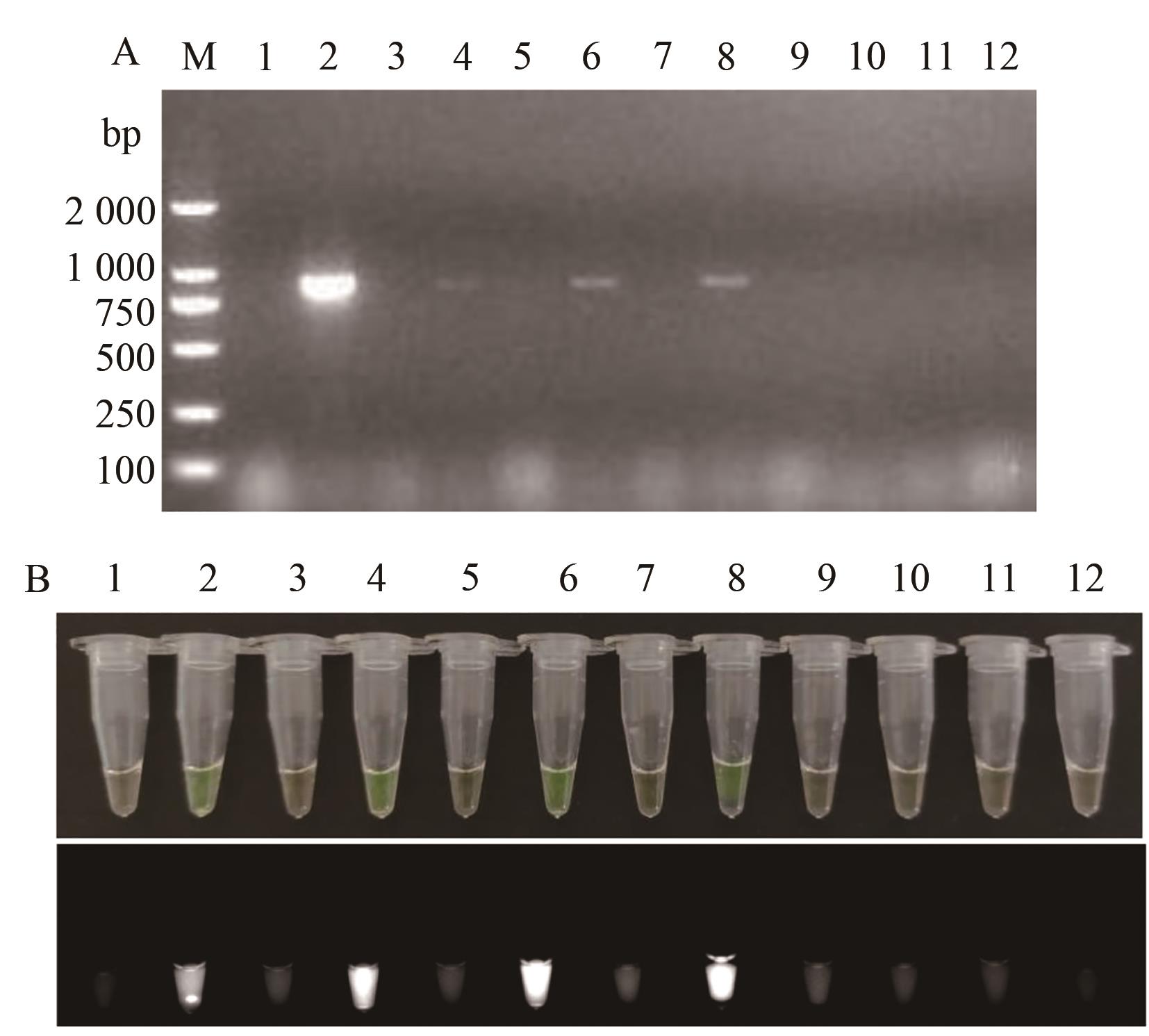
图10 TSWV RT-LAMP和RT-PCR田间样品检测结果A:凝胶电泳;B:荧光可视化结果;M—2 000 DNA marker;1—阴性对照;2—阳性对照;3~12—田间烟草叶片样品
Fig. 10 Results of TSWV RT-LAMP and RT-PCR field samplesA: Gel electrophoresis; B: Fluorescence visualization results; M—2 000 DNA marker; 1—Negative control; 2—Positive control; 3~12—Field tobacco leaf samples
| 1 | GOLDBACH R, PETER D. Molecular and Biological Aspects of Tospoviruses [M]. New York:Springer Science,Business Media New York,1996:129-157. |
| 2 | SCHOLTHOF K B G, ADKINS S, CZOSNEK H, et al.. Top 10 plant viruses in molecular plant pathology [J]. Mol. Plant Pathol., 2011,12(9): 938-954. |
| 3 | KORMELINK R, DE H P, MEURS C, et al.. The nucleotide sequence of the M RNA segment of tomato spotted wilt virus, a bunyavirus with two ambisense RNA segments [J]. J. Gen. Virol., 1993,73(11): 2795-2804. |
| 4 | KATO K, HANDA K, KAMEYA I M. Melon yellow spot virus: a distinct species of the genus tospovirus isolated from melon [J]. Phytopathology, 2000,90(4):422-426. |
| 5 | FAUQUET C M, MAYO M A, MANILOFF J, et al.. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses [R]. Cambridge: Academic Press Cambridge, 2005. |
| 6 | 刘勇, 李凡, 李月月, 等. 侵染我国主要蔬菜作物的病毒种类、分布与发生趋势 [J]. 中国农业科学, 2019,52(2):239-261. |
| LIU Y, LI F, LI Y Y, et al.. Identification, distribution and occurrence of viruses in the main vegetables of China [J]. Sci. Agric. Sin., 2019, 52(2): 239-261. | |
| 7 | PARRELLA G, GOGNALONS P, GEBRE S K, et al.. An update of the host range of tomato spotted wilt virus [J]. J. Plant Pathol., 2003,85(4):227-264. |
| 8 | 康华军, 李建设, 高艳明, 等. 宁夏银川地区番茄斑萎病毒和南方番茄病毒复合侵染分子鉴定[J]. 中国蔬菜, 2022(4):29-34. |
| KANG H J, LI J S, GAO Y M, et al.. Molecular identification of co-infection of tomato spotted wilt virus and Southern tomato virus in Yinchuan region of Ningxia [J]. China Veget., 2022(4):29-34. | |
| 9 | 陈东亮, 李明远, 李雪梅, 等. 北京地区莴苣中番茄斑萎病毒的鉴定[J]. 中国蔬菜, 2018(1):65-69. |
| CHEN D L, LI M Y, LI X M, et al.. Identification of tomato spotted wilt virus from lettuce in Beijing [J]. China Veget., 2018(1):65-69. | |
| 10 | XIAO L, LI Y Y, LAN P X, et al.. First report of tomato spotted wilt virus infecting cowpea in China [J/OL]. Plant Dis., 2016,100(1):233 [2023-04-23]. . |
| 11 | PRINS M, GOLDBACH R. The emerging problem of tospovirus infection and nonconventional methods of control [J]. Trends Microbiol., 1998,6(1):31-35. |
| 12 | TOMARU K, KUBO S, TAKANAMI Y. Occurrence of tomato spotted wilt virus in tobacco plants in Ryukyu islands [J]. Jpn. J. Phytopathol., 1982,48(3):336-339. |
| 13 | BLANCARD D. Tomato Diseases [M]. Cambridge: Academic Press Cambridge, 2012:1-688. |
| 14 | 许泽永, REDDY D V R, RAJESHWARI R, 等. 我国南方花生发生一种由蕃茄斑萎病毒引起的新病害[J]. 病毒学报, 1986,2(3):271-274, 295. |
| XU Z Y, REDDY D V R, RAJESHWARI R, et al.. A new peanut virus disease caused by tomato spotted wilt virus in southern China [J]. Chin. J. Virol., 1986,2(3):271-274, 295. | |
| 15 | 苏大昆, 袁宣泽, 谢永红, 等. 成都、渡口两市番茄中检出番茄斑萎病毒[J]. 植物病理学报, 1987,17(4):255-256. |
| SU D K, YUAN X Z, XIE Y H, et al.. Tomato spotted wilt virus in tomato in Chengdu and Dukou [J]. Acta Phytopathol. Sci., 1987, 17(4):255-256. | |
| 16 | 姚革. 四川晒烟上发现番茄斑萎病毒(TSWV) [J]. 中国烟草, 1992(4):2-4. |
| YAO G. Tomato spotted wilt virus (TSWV) found on sun-dried tobacco in Sichuan [J]. Chin. Tab. Sci., 1992(4):2-4. | |
| 17 | 张仲凯, 丁铭, 方琦, 等. 番茄斑萎病毒属(Tospovirus)病毒在云南的发生分布研究初报[J]. 西南农业学报, 2004,17():163-168. |
| ZHANG Z K, DING M, FANG Q, et al.. The preliminary study of the occurrence and distribution of Tospovirus in Yunnan [J]. Southwest Chin. J. Agric. Sci., 2004, 17(S1):163-168. | |
| 18 | GILBERTSON R L, BATUMAN O, WEBSTER C G, et al.. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses [J]. Annu. Rev. Virol., 2015,2:67-93. |
| 19 | 刘雅婷, 郑元仙, 李永忠, 等. 番茄斑萎病毒在烟草植株上症状学特征[J]. 中国农学通报, 2009,25(19):190-193. |
| LIU Y T, ZHENG Y X, LI Y Z, et al.. Symptomology of tomato spotted wilt virus on tobacco [J]. Chin. Agric. Sci. Bull., 2009, 25(19):190-193. | |
| 20 | SEVIK M A, ARLI S M. Estimation of the effect of tomato spotted wilt virus (TSWV) infection on some yield components of tomato [J]. Phytoparasitica, 2012,40(1):87-93. |
| 21 | 佟爱仔, 赵兴能, 孔宝华, 等. 侵染云南烟草的番茄斑萎病毒(TSWV)的RT-PCR检测[J]. 云南农业大学学报(自然科学), 2012,27(6):809-813. |
| TONG A Z, ZHAO X N, KONG B H, et al.. The detection of tomato spotted wilt virus from tobacco by RT-PCR in Yunnan [J]. J. Yunnan Agric. Univ.( Nat. Sci.), 2012,27(6):809-813. | |
| 22 | NOTOMI T O H M. Loop-mendiated isothermal amplifaction of DNA [J]. Nucl. Acids Res., 2000, 12(28): 63-63. |
| 23 | FUKUTA S, IIDA T, MIZUKAMI Y, et al.. Detection of Japanese yam mosaic virus by RT-LAMP [J]. Arch. Virol., 2003,148:1713-1720. |
| 24 | HUANG W E, LIM B, HSU C C, et al.. RT‐LAMP for rapid diagnosis of coronavirus SARS‐CoV‐2 [J]. Microb. Biotechnol., 2020,13(4):950-961. |
| 25 | CHOI G, MOEHLING T J, MEAGHER R J. Advances in RT-LAMP for COVID-19 testing and diagnosis [J]. Expert Rev. Mol. Diagn., 2023,23(1):9-28. |
| 26 | ZHOU L, CHEN Y, FANG X, et al.. Microfluidic-RT-LAMP chip for the point-of-care detection of emerging and re-emerging enteric coronaviruses in swine [J]. Anal. Chim. Acta., 2020,1125:57-65. |
| 27 | LI X, HU W, LI Y, et al.. Development of an RT-LAMP assay for the detection of maize yellow mosaic virus in maize [J/OL]. J. Virol. Methods, 2022, 300: 114384 [2023-04-23]. . |
| 28 | ESTRELA P F N, DE M M G, DE O K G, et al.. Ten-minute direct detection of Zika virus in serum samples by RT-LAMP [J/OL]. J. Virol. Methods, 2019, 271: 113675 [2023-04-23]. . |
| 29 | VERMA G, RAIGOND B, PATHANIA S, et al.. Development and comparison of reverse transcription-loop-mediated isothermal amplification assay (RT-LAMP), RT-PCR and real time PCR for detection of potato spindle tuber viroid in potato [J]. Eur. J. Plant Pathol., 2020,158:951-964. |
| 30 | FUKUTA S, OHISHI K, YOSHIDA K, et al.. Development of immunocapture reverse transcription loop-mediated isothermal amplification for the detection of tomato spotted wilt virus from chrysanthemum [J]. J. Virol. Methods, 2004,121(1):49-55. |
| 31 | MEENA P N, KHARBIKAR L L, RANA R S, et al.. Detection of Mesta yellow vein mosaic virus (MeYVMV) in field samples by a loop-mediated isothermal amplification reaction [J]. J. Virol. Methods, 2019,263:81-87. |
| 32 | KHAN M, WANG R, LI B, et al.. Comparative evaluation of the LAMP assay and PCR-based assays for the rapid detection of Alternaria solani [J/OL]. Front. Microbiol., 2018,9:2089 [2023-04-23]. . |
| 33 | 张雯娜, 李晋玉, 田金艳, 等. 逆转录环介导等温扩增技术快速检测大豆花叶病毒[J]. 大豆科学, 2014,33(3):422-428. |
| ZHANG W N, LI J Y, TIAN J Y, et al.. Rapid detection of soybean mosaic virus by reverse-transcription loop-mediated isothermal amplification [J]. Soybean Sci., 2014,33(3):422-428. | |
| 34 | LE D T, NETSU O, UEHARA I T, et al.. Molecular detection of nine rice viruses by a reverse-transcription loop-mediated isothermal amplification assay [J]. J. Virol. Methods, 2010,170(1-2):90-93. |
| 35 | ZHANG Q, STANDISH I, WINTERS A D, et al.. Development and evaluation of reverse transcription loop-mediated isothermal amplification assay for the detection of the Fathead minnow nidovirus [J]. J. Virol. Methods, 2014,202:39-45. |
| 36 | LIU Y, WANG Z, QIAN Y, et al.. Rapid detection of tobacco mosaic virus using the reverse transcription loop-mediated isothermal amplification method [J]. Arch. Virol., 2010,155(10):1681-1685. |
| 37 | 周彤, 杜琳琳, 范永坚, 等. 水稻黑条矮缩病毒RT-LAMP快速检测方法的建立[J]. 中国农业科学, 2012,45(7):1285-1292. |
| ZHOU T, DU L L, FAN Y J, et al.. Development of a RT-LAMP assay for rapid detection of rice black-streaked dwarf virus [J]. Sci. Agric. Sin., 2012, 45(7):1285-1292. | |
| 38 | 高彦萍, 吕和平, 张武, 等. 马铃薯卷叶病毒RT-LAMP检测方法的建立[J]. 核农学报, 2020,34(9):1943-1950. |
| GAO Y P, LYU H P, ZHANG W, et al.. Establishment of the detection method for potato leafroll virus by RT-LAMP [J]. J. Nucl. Agric. Sci., 2020, 34(9):1943-1950. | |
| 39 | 黄丽, 苏华楠, 唐科志, 等. 柑橘黄龙病LAMP快速检测方法的建立及应用[J]. 果树学报, 2012,29(6):1121-1126. |
| HUANG L, SU H N, TANG K Z, et al.. Establishment and application of loop-mediated isothermal amplification assay for the detection of Citrus huanglongbing [J]. J. Fruit Sci., 2012, 29(6):1121-1126. | |
| 40 | PU R Y, LIU S, REN X Y, et al.. The screening value of RT-LAMP and RT-PCR in the diagnosis of COVID-19: systematic review and meta-analysis [J/OL]. J. Virol. Methods, 2022,300:114392 [2023-04-23]. . |
| 41 | SUZUKI R, FUKUTA S, MATSUMOTO Y, et al.. Development of reverse transcription loop-mediated isothermal amplification assay as a simple detection method of Chrysanthemum stem necrosis virus in chrysanthemum and tomato [J]. J. Virol. Methods, 2016,236:29-34. |
| 42 | 高彦萍, 张武, 王国祥, 等. 马铃薯卷叶病毒PLRV RT-LAMP检测方法优化[J]. 植物保护, 2019,45(6):259-264. |
| GAO Y P, ZHANG W, WANG G X, et al.. Optimization of the PLRV RT-LAMP detection method [J]. Plant Prot., 2019,45(6):259-264. | |
| 43 | 王贤达, 林雄杰, 胡菡青, 等. 柑橘黄龙病可视化LAMP检测技术的建立及应用[J]. 热带作物学报, 2014,35(5):918-924. |
| WANG X D, LIN X J, HU H Q, et al.. Establishment and application of visual loop-mediated amplification assay on citrus huanglongbing [J]. Chin. J. Trop. Crops, 2014,35(5):918-924. | |
| 44 | 陈海, 申亚南, 吕文竹, 等. 甘蔗高粱花叶病毒RT-LAMP快速检测方法的建立及评价[J]. 分子植物育种, 2020,18(10):3282-3287. |
| CHEN H, SHEN Y N, LYU W Z, et al.. Establishment and evaluation of RT-LAMP for the rapid detection of sorghum mosaic virus [J]. Mol. Plant Breeding, 2020,18(10):3282-3287. |
| [1] | 张笑笑, 李晓倩, 朱诚, 吕晨泽. 刀豆凝集素快速检测技术的研究现状及发展趋势[J]. 中国农业科技导报, 2024, 26(6): 214-225. |
| [2] | 李培武,丁小霞. 我国粮油质量安全防控技术研究与发展对策[J]. , 2011, 13(5): 54-58. |
| [3] | 汪笑宇,周遵春,关晓燕,姜北,陈仲,董颖,杨爱馥. 仿刺参及养殖环境中溶藻弧菌和灿烂弧菌的PCR快速检测[J]. , 2010, 12(3): 125-130. |
| [4] | 马妍,李健,王群,何玉英,王斌. 建立PC-PCR法快速、半定量检测养殖环境和水产动物中的致病性副溶血弧菌[J]. , 2008, 10(S1): 67-72. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号