




















Journal of Agricultural Science and Technology ›› 2024, Vol. 26 ›› Issue (9): 54-61.DOI: 10.13304/j.nykjdb.2023.0176
• BIOTECHNOLOGY & LIFE SCIENCE • Previous Articles Next Articles
Received:2023-03-13
Accepted:2023-11-22
Online:2024-09-15
Published:2024-09-13
作者简介:李丹E-mail: lidans10@126.com
基金资助:CLC Number:
Dan LI. Study on Folding of SprD Protein Mediated by Pro-peptide in vitro[J]. Journal of Agricultural Science and Technology, 2024, 26(9): 54-61.
李丹. 前导肽介导的SprD蛋白体外折叠研究[J]. 中国农业科技导报, 2024, 26(9): 54-61.
引物名称 Primer name | 引物序列 Primer sequence (5’ - 3’) |
|---|---|
| pro-sprD sense | |
| pro-sprD anti | |
| Y-1D’ sense | GATCACGTTGCACAAGCGGCGCAGTCCGTGCCTTAC |
| Y-1D’ anti | GGCACGGACTGCGCCGCTTGTGCAACGTGATC |
| G-44D sense | GTCATTTCTGAAAAAGACGGGAAAGTGCAAAAGC |
| G-44D anti | GCTTTTGCACTTTCCCGTCTTTTTCAGAAATGAC |
| I-48V sense | CCAAGAAAAAAGATGTC GTTTCTGAAAAAGGCG |
| I-48V anti | CGCCTTTTTCAGAAAC GACATCTTTTTTCTTGG |
| E112A sense | CGGAATTGCGTGGGCGATCGCAAACAATATC |
| E112A anti | GCGATCGCCCACGCAATTCCGTTAATGATC |
| S221A sense | GTACAATGGTACG GCAATGGCATCTCCGCACG |
| S221A anti | GCGGAGATGC CATTGCCGTACCATTGTACG |
| S221C sense | GTGCGGAGATGCCAT ACACGTACCATTGTACGCGC |
| S221C anti | GTACAATGGTACGTGT ATGGCATCTCCGCACGT |
Table 1 Primers used in the study
引物名称 Primer name | 引物序列 Primer sequence (5’ - 3’) |
|---|---|
| pro-sprD sense | |
| pro-sprD anti | |
| Y-1D’ sense | GATCACGTTGCACAAGCGGCGCAGTCCGTGCCTTAC |
| Y-1D’ anti | GGCACGGACTGCGCCGCTTGTGCAACGTGATC |
| G-44D sense | GTCATTTCTGAAAAAGACGGGAAAGTGCAAAAGC |
| G-44D anti | GCTTTTGCACTTTCCCGTCTTTTTCAGAAATGAC |
| I-48V sense | CCAAGAAAAAAGATGTC GTTTCTGAAAAAGGCG |
| I-48V anti | CGCCTTTTTCAGAAAC GACATCTTTTTTCTTGG |
| E112A sense | CGGAATTGCGTGGGCGATCGCAAACAATATC |
| E112A anti | GCGATCGCCCACGCAATTCCGTTAATGATC |
| S221A sense | GTACAATGGTACG GCAATGGCATCTCCGCACG |
| S221A anti | GCGGAGATGC CATTGCCGTACCATTGTACG |
| S221C sense | GTGCGGAGATGCCAT ACACGTACCATTGTACGCGC |
| S221C anti | GTACAATGGTACGTGT ATGGCATCTCCGCACGT |
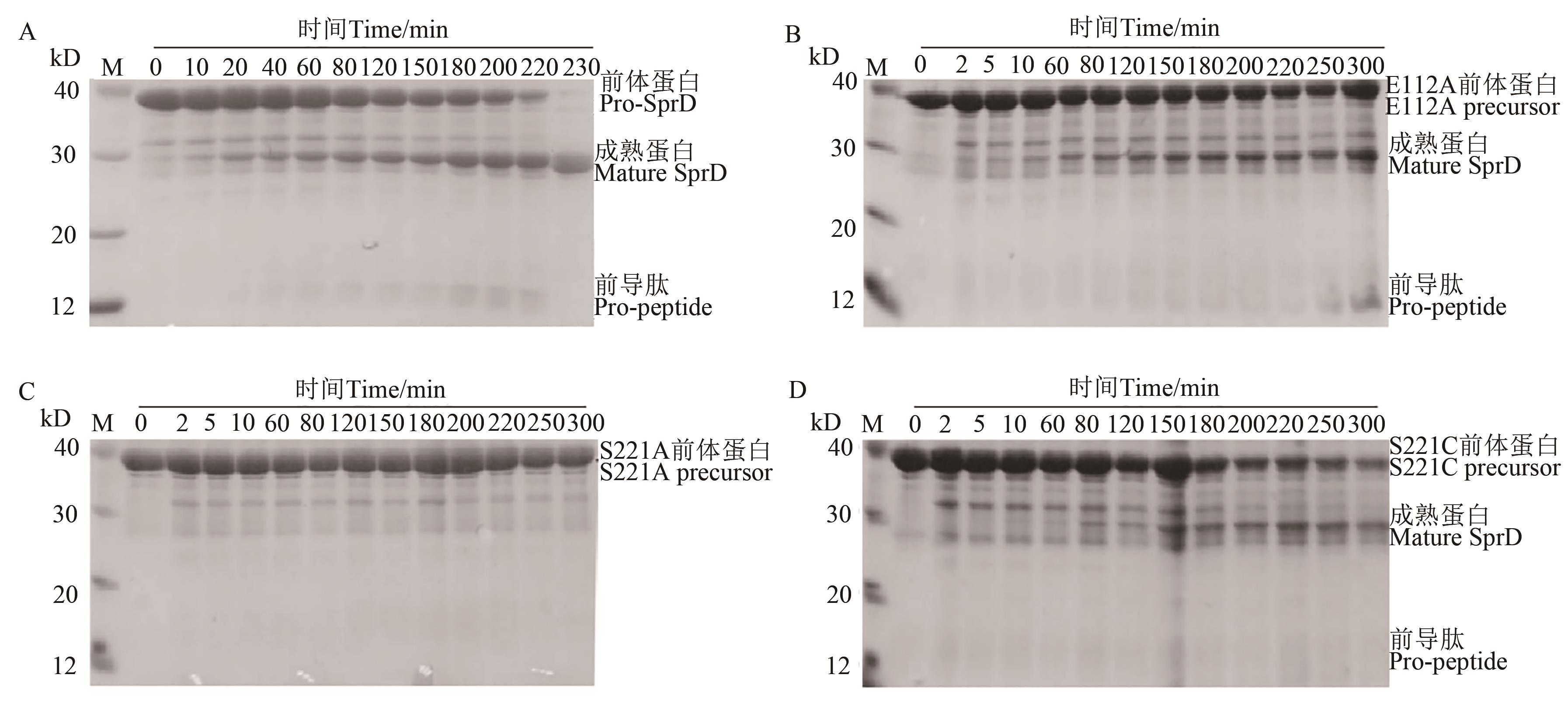
Fig. 2 Processing of wild-type precursors to mature enzymes in vitroA: Folding and maturation of wild-type precursor; B~D: Folding and maturation of E112A, S221A and S221C precursors
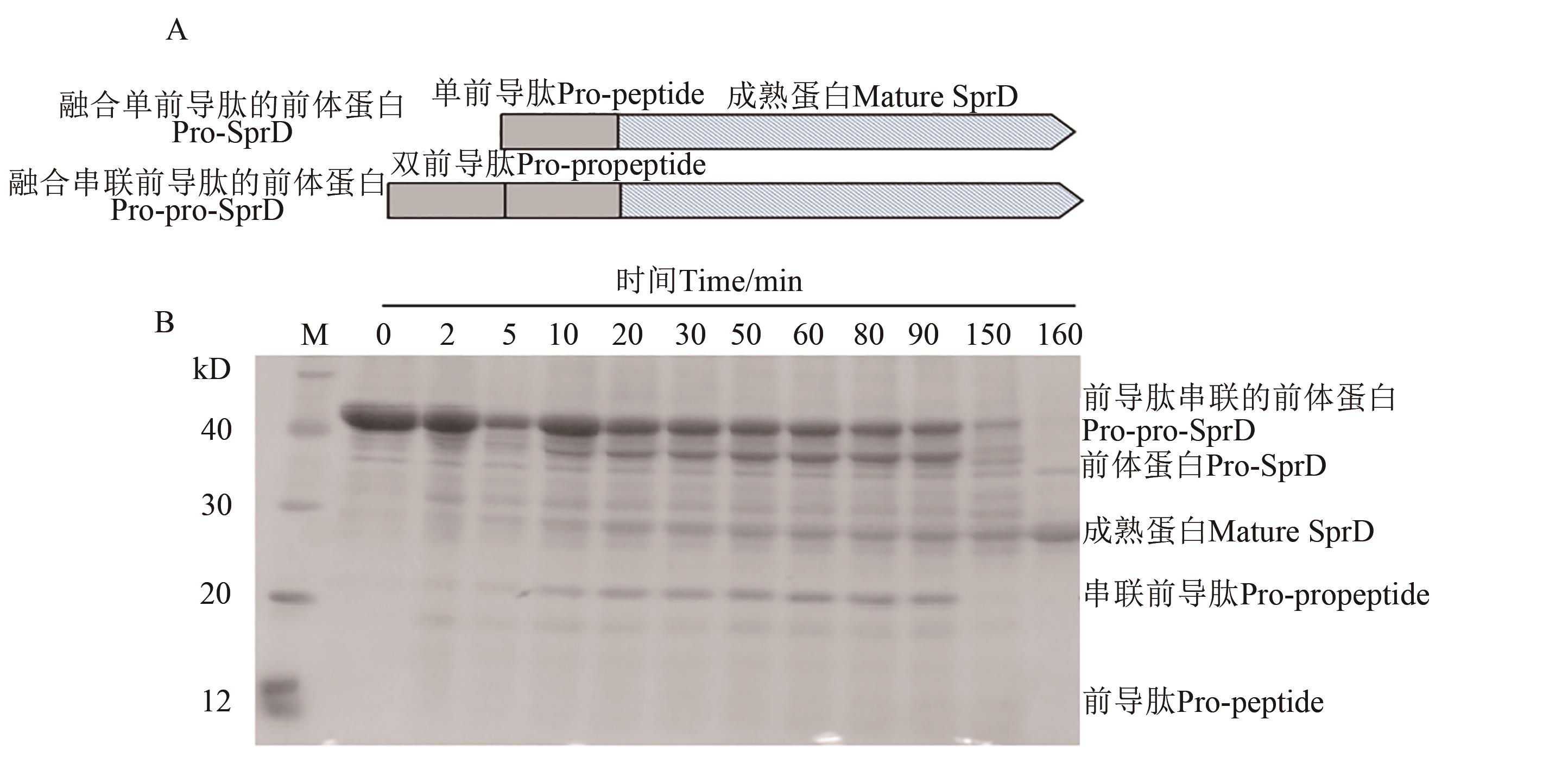
Fig. 3 Maturation process of pro-pro-SprD precursorA: Schematic of tandem expression of pro-peptides; B: Processing and maturation of pro-pro-SprD precursors
| 前体蛋白Precursor | 突变范围 Mutation range | 成熟时间 Maturation time/min | 米氏常数 Km/(μmol·L-1) | 催化常数 kcat/(s-1) | 催化常数/米氏常数 kcat/Km/(s-1·mmol-1·L) |
|---|---|---|---|---|---|
| WT | 无 No | 230~240 | 75.69±12.00 | 47.02±7.00 | 621 |
| I-48V | 前导肽 Pro-peptide | 200~210 | 57.68±11.00 | 46.53±6.00 | 807 |
| G-44D | 前导肽 Pro-peptide | 210~220 | 80.45±13.00 | 10.55±3.00 | 131 |
| Y-1D’ | 前导肽 Pro-peptide | 80~90 | 66.68±10.00 | 45.19±5.00 | 678 |
Table 2 Catalytic properties of active enzymes
| 前体蛋白Precursor | 突变范围 Mutation range | 成熟时间 Maturation time/min | 米氏常数 Km/(μmol·L-1) | 催化常数 kcat/(s-1) | 催化常数/米氏常数 kcat/Km/(s-1·mmol-1·L) |
|---|---|---|---|---|---|
| WT | 无 No | 230~240 | 75.69±12.00 | 47.02±7.00 | 621 |
| I-48V | 前导肽 Pro-peptide | 200~210 | 57.68±11.00 | 46.53±6.00 | 807 |
| G-44D | 前导肽 Pro-peptide | 210~220 | 80.45±13.00 | 10.55±3.00 | 131 |
| Y-1D’ | 前导肽 Pro-peptide | 80~90 | 66.68±10.00 | 45.19±5.00 | 678 |

Fig. 6 Precursor proteins affect the activity of mature enzymesA: Precursors inhibited the action of active enzymes; B: Relative inhibition of precursors on enzymes
| 1 | CONTESINI F J, MELO R R, SATO H H. An overview of Bacillus proteases: from production to application [J]. Crit. Rev. Biotechnol., 2018, 38(3): 321-334. |
| 2 | BALLINGER M D, TOM J, WELLS J A. Designing subtilisin BPN’ to cleave substrates containing dibasic residues [J]. Biochemistry, 1995, 34(41): 13312-13319. |
| 3 | IKEMURA H, INOUYE M. In vitro processing of pro-subtilisin in Escherichia coli [J]. J. Biol. Chem., 1988, 263(26): 12959-12963. |
| 4 | CHO J S, OH H J, JANG Y E, et al.. Synthetic pro-peptide design to enhance the secretion of heterologous proteins by Saccharomyces cerevisiae [J/OL]. Microbiol. Open, 2022, 11(3): e1300 [2023-02-12]. . |
| 5 | PENG Z, ZHANG J, SONG Y, et al.. Engineered pro-peptide enhances the catalytic activity of keratinase to improve the conversion ability of feather waste [J]. Biotechnol. Bioeng., 2021, 118(7): 2559-2571. |
| 6 | 罗文. 前导肽对米黑根毛霉脂肪酶的别构调控及其影响机理探究[D]. 广州: 华南农业大学, 2018. |
| LUO W. The allosteric regulation mechanism of Rhizomucor miehei lipase via modification of the propeptide [D]. Guangzhou: South China Agricultural University, 2018. | |
| 7 | 刘柏宏. Bacillus licheniformis角蛋白酶的高效表达、热稳定性及底物特异性改造[D]. 无锡: 江南大学, 2015. |
| LIU B H. Over expression of Bacillus licheniformis keratinase, its molecular modification for enhanced thermostability and substrate specificity [D]. Wuxi: Jiangnan University, 2015. | |
| 8 | INOUYE M, FU X, SHINDE U. Substrate-induced activation of a trapped IMC-mediated protein folding intermediate [J]. Nat. Struct. Mol. Biol., 2001, 8(4): 321-325. |
| 9 | 刘海燕, 张荣珍, 李利宏, 等. 前导肽对Aspergillus pseudoglaucus酸性蛋白酶App表达及功能的影响[J]. 食品与生物技术学, 2020, 39(3): 32-40. |
| LIU H Y, ZHANG R Z, LI L H, et al.. Effects of propeptide on the expression and enzyme function of Aspergillus pseudoglaucus aspartic protease App [J]. J. Food Sci. Biotechnol., 2020, 39(3): 32-40. | |
| 10 | NAGAYAMA M, MAEDA H, KURODA K, et al.. Mutated intramolecular chaperones generate high-activity isomers of mature enzymes [J]. Biochemistry, 2012, 51(17): 3547-3553. |
| 11 | 李丹, 黄非, 夏梦芸, 等. 一新中温碱性蛋白酶基因的克隆及原核表达[J]. 微生物学报, 2013, 53(11): 1240-1250. |
| LI D, HUANG F, XIA M Y, et al.. Molecular cloning and expression of a novel mesophilic alkaline protease from Bacillus sp. L010 in Escherichia coli [J]. Acta Microbiol. Sin., 2013, 53(11): 1240-1250. | |
| 12 | KOSCHORRECK K, SCHMID R D, URLACHER V B. Improving the functional expression of a Bacillus licheniformis laccase by random and site-directed mutagenesis [J/OL]. BMC Biotechnol., 2009, 9:12 [2023-02-12]. . |
| 13 | TAKAGI H, ARAFUKA S, INOUYE M, et al.. The effect of amino acid deletion in subtilisin E, based on structural comparison with a microbial alkaline elastase, on its substrate specificity and catalysis [J]. J. Biochem., 1992, 111(5): 584-588. |
| 14 | LI Y, HU Z, JORDAN F, et al.. Functional analysis of the propeptide of subtilisin E as an intramolecular chaperone for protein folding [J]. J. Biolog. Chem., 1995, 270(42): 25127-25132. |
| 15 | JUNIOR N, CARDOSO M H, CANDIDO E S, et al.. An acidic model pro-peptide affects the secondary structure, membrane interactions and antimicrobial activity of a crotalicidin fragment [J/OL]. Sci. Rep., 2018, 8(1): 11127 [2023-02-12]. . |
| 16 | BALTULIONIS G, BLIGHT M, ROBIN A, et al.. The role of propeptide-mediated autoinhibition and intermolecular chaperone in the maturation of cognate catalytic domain in leucine aminopeptidase [J]. J. Struct. Biol., 2021, 213(3): 107741-107753. |
| 17 | LILIE H, SCHWARZ E, RUDOLPH R. Advances in refolding of proteins produced in E. coli [J]. Curr. Opin. Biotech., 1998, 9(5): 497-501. |
| 18 | KAUR J, SINGH A, PANDA A K, et al.. Protocol for in-vitro purification and refolding of hexachlorocyclohexane degrading enzyme haloalkane dehalogenase LinB from inclusion bodies [J/OL]. Enzyme Microb. Technol., 2021, 146:109760 [2023-02-12]. . |
| 19 | YABUTA Y, TAKAGI H, INOUYE M, et al.. Folding pathway mediated by an intramolecular chaperone: propeptide release modulates activation precision of pro-subtilisin [J]. J. Biol. Chem., 2001, 276(48): 44427-44434. |
| 20 | TAKAGI H, OHTSU I, NAKAMORI S. Construction of novel subtilisin E with high specificity, activity and productivity through multiple amino acid substitutions [J]. Protein Eng., 1997, 10(9): 985-989. |
| 21 | ISHIDA K, SHIMIZU M, WAKASUGI A, et al.. Development of a novel peptide inhibitor of subtilisin BPN’ [J]. FEBS Open Bio., 2022, 12(11): 2057-2064. |
| 22 | BRYAN N B. Protein engineering of subtilisin [J]. Biochim. Biophys. Acta, 2000, 1543(2): 203-222. |
| 23 | FU X, INOUYE M, SHINDE U. Folding pathway mediated by an intramolecular chaperone: the inhibitory and chaperone functions of the subtilisin propeptide are not obligatorily linked [J]. J. Biol. Chem., 2000, 275(22): 16871-16878. |
| 24 | JAIN S C, SHINDE U, LI Y, et al.. The crystal structure of an autoprocessed Ser221Cys-subtilisin E-propeptide complex at 2.0A˚ resolution [J]. J. Mol. Biol., 1998, 284(1): 137-144. |
| 25 | SU C, GONG J S, SUN Y X, et al.. Combining pro-peptide engineering and multisite saturation mutagenesis to improve the catalytic potential of keratinase [J]. ACS Synth. Biol., 2019, 8(2):425-433. |
| 26 | OUYANG X, LIU Y, QU R, et al.. Optimizing protein-glutaminase expression in Bacillus subtilis [J]. Curr. Microbiol., 2021, 78(5):1752-1762. |
| 27 | 彭政. 芽孢杆菌降解羽毛角蛋白关键步骤解析及角蛋白酶的高效表达[D]. 无锡: 江南大学, 2020. |
| PENG Z. Analysis of key steps in degradation of feather keratin by Bacillus and efficient expression of keratinase [D]. Wuxi: Jiangnan University, 2020. | |
| 28 | 沈卫锋, 牛宝龙, 翁宏飚, 等. 枯草芽孢杆菌作为外源基因表达系统的研究进展[J]. 浙江农业学报, 2005, 17(4):234-238. |
| SHEN W F, NIU B L, WENG H B, et al.. The studies on Bacillus subtilis as an expression system of foreign genes [J]. Acta Agric. Zhejiangensis, 2005, 17(4):234-238. |
| [1] | Zequn LU, Ning LIU, Honglian ZHANG, Yuan WANG, Huoqing HUANG. Improvement of Heterologous Protein Secretion and Folding Pathways of Pichia pastoris [J]. Journal of Agricultural Science and Technology, 2024, 26(1): 18-27. |
| [2] | ZHENG Xiao-mei, CHU Xiao-yu, WU Ning-feng, FAN Yun-liu. Steric Chaperone-Lipase Specific Foldase [J]. , 2011, 13(6): 66-71. |
| [3] | YANG Mei-ling, ZHAO Xi-an, LIU Dong-jun, CANG Ming. Expression of Interleukin-6 and Its Receptor mRNA in Sheep Oocytes Maturation Process [J]. , 2009, 11(5): 71-76. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号 