




















Journal of Agricultural Science and Technology ›› 2022, Vol. 24 ›› Issue (9): 79-87.DOI: 10.13304/j.nykjdb.2022.0297
• BIOTECHNOLOGY & LIFE SCIENCE • Previous Articles Next Articles
Yue WU1,2( ), Yun’an WANG1,2(
), Yun’an WANG1,2( ), Ha’nan SONG2, Weijun GUAN2, Nan LI1(
), Ha’nan SONG2, Weijun GUAN2, Nan LI1( )
)
Received:2022-04-13
Accepted:2022-06-07
Online:2022-09-15
Published:2022-10-11
Contact:
Nan LI
吴月1,2( ), 王育南1,2(
), 王育南1,2( ), 宋哈楠2, 关伟军2, 李楠1(
), 宋哈楠2, 关伟军2, 李楠1( )
)
通讯作者:
李楠
作者简介:吴月 E-mail:wuyueyouxiang0320@163.com基金资助:CLC Number:
Yue WU, Yun’an WANG, Ha’nan SONG, Weijun GUAN, Nan LI. Primary Culture and Differentiation Potential of Mesenchymal Stem Cells from Gushi Chicken Umbilical Cord[J]. Journal of Agricultural Science and Technology, 2022, 24(9): 79-87.
吴月, 王育南, 宋哈楠, 关伟军, 李楠. 固始鸡脐带间充质干细胞原代培养及其诱导分化潜能研究[J]. 中国农业科技导报, 2022, 24(9): 79-87.
基因名称 Gene name | 引物序列 Primer sequence (3′-5′) | 目的片段长度 Target segment length/bp | 退火温度 Annealing temperature/℃ |
|---|---|---|---|
| GAPDH | F:GGACGCTGGGATGATGTTCT R:GGGTGGTGCTAAGCGTGTTA | 292 | 58.7 |
| CD29 | F:TGAATGACACGCAGGAAGATGGAAG R:AGGCAGGGCTGTTAGGTTCTCC | 219 | 59.3 |
| CD44 | F:CCTCCTGGCACTGGCATTGATC R:CACTCCACTCTTCATGTCACCATCC | 271 | 58.9 |
| CD73 | F:GGCATCGTTGGCTACACTACACAG R:GCTTGTACCACTGGCACCTTCC | 326 | 54.2 |
| CD90 | F:ACCAAGGACAACAGGAAGCACATC R:GGTGTTCTGGATCAAGAGGCTGAAG | 258 | 57.8 |
| CD166 | F:ACCAGCAGTGCAATGGACAGTTAC R:GCAACCAGCAGAAGACCGACTAC | 294 | 58.3 |
| CD34 | F:GCCACTCGCATCCAGAGAACAC R:GTCAGCATCCCCTTCGCATATCG | 326 | 61.4 |
| CD45 | F:GTCATGGTTACTCGCTGTGAGGAAG R:CGTCGGAGTTTGAGAAGGAGATGTG | 263 | 58.7 |
| ACAN | F:TGGTGTTGATGGAAGTGGTG R:GTGGGAAAGCCTGAGGTGAG | 336 | 60.4 |
| SOX9 | F:GCTCGAAGGAAGCTGGCTGA R:ATGGCGTTGGGGGAGATGTG | 254 | 59.7 |
| LPL | F:GTGACCAAGGTAGACCAGCCATTC R:TCGCCTGACTTCACTCTGACTCTC | 248 | 54.3 |
| PPAR-γ | F:CGAATGCCACAAGCGGAGAAGG R:CACTGCCTCCACAGAGCGAAAC | 330 | 59.6 |
| collagen type Ⅰ | F:GGTAAGGATGGTCGCAATGGTCTC R:GGGTCAGCAGGGTCTCAATTTGG | 256 | 59.1 |
| osteopontin | F:CCTGACATTCCTAGCAAGAGCCAAG R:CTGTCTCCTCCGTCCACCTCAG | 398 | 58.9 |
Table 1 Table for primer information
基因名称 Gene name | 引物序列 Primer sequence (3′-5′) | 目的片段长度 Target segment length/bp | 退火温度 Annealing temperature/℃ |
|---|---|---|---|
| GAPDH | F:GGACGCTGGGATGATGTTCT R:GGGTGGTGCTAAGCGTGTTA | 292 | 58.7 |
| CD29 | F:TGAATGACACGCAGGAAGATGGAAG R:AGGCAGGGCTGTTAGGTTCTCC | 219 | 59.3 |
| CD44 | F:CCTCCTGGCACTGGCATTGATC R:CACTCCACTCTTCATGTCACCATCC | 271 | 58.9 |
| CD73 | F:GGCATCGTTGGCTACACTACACAG R:GCTTGTACCACTGGCACCTTCC | 326 | 54.2 |
| CD90 | F:ACCAAGGACAACAGGAAGCACATC R:GGTGTTCTGGATCAAGAGGCTGAAG | 258 | 57.8 |
| CD166 | F:ACCAGCAGTGCAATGGACAGTTAC R:GCAACCAGCAGAAGACCGACTAC | 294 | 58.3 |
| CD34 | F:GCCACTCGCATCCAGAGAACAC R:GTCAGCATCCCCTTCGCATATCG | 326 | 61.4 |
| CD45 | F:GTCATGGTTACTCGCTGTGAGGAAG R:CGTCGGAGTTTGAGAAGGAGATGTG | 263 | 58.7 |
| ACAN | F:TGGTGTTGATGGAAGTGGTG R:GTGGGAAAGCCTGAGGTGAG | 336 | 60.4 |
| SOX9 | F:GCTCGAAGGAAGCTGGCTGA R:ATGGCGTTGGGGGAGATGTG | 254 | 59.7 |
| LPL | F:GTGACCAAGGTAGACCAGCCATTC R:TCGCCTGACTTCACTCTGACTCTC | 248 | 54.3 |
| PPAR-γ | F:CGAATGCCACAAGCGGAGAAGG R:CACTGCCTCCACAGAGCGAAAC | 330 | 59.6 |
| collagen type Ⅰ | F:GGTAAGGATGGTCGCAATGGTCTC R:GGGTCAGCAGGGTCTCAATTTGG | 256 | 59.1 |
| osteopontin | F:CCTGACATTCCTAGCAAGAGCCAAG R:CTGTCTCCTCCGTCCACCTCAG | 398 | 58.9 |

Fig. 4 Detection of Gushi chicken UCMSCs markers by immunofluorescence stainingNote: DAPI—Stained nuclei; FITC—Immunofluorescence; Merge—Merged image of DAPI and FITC.
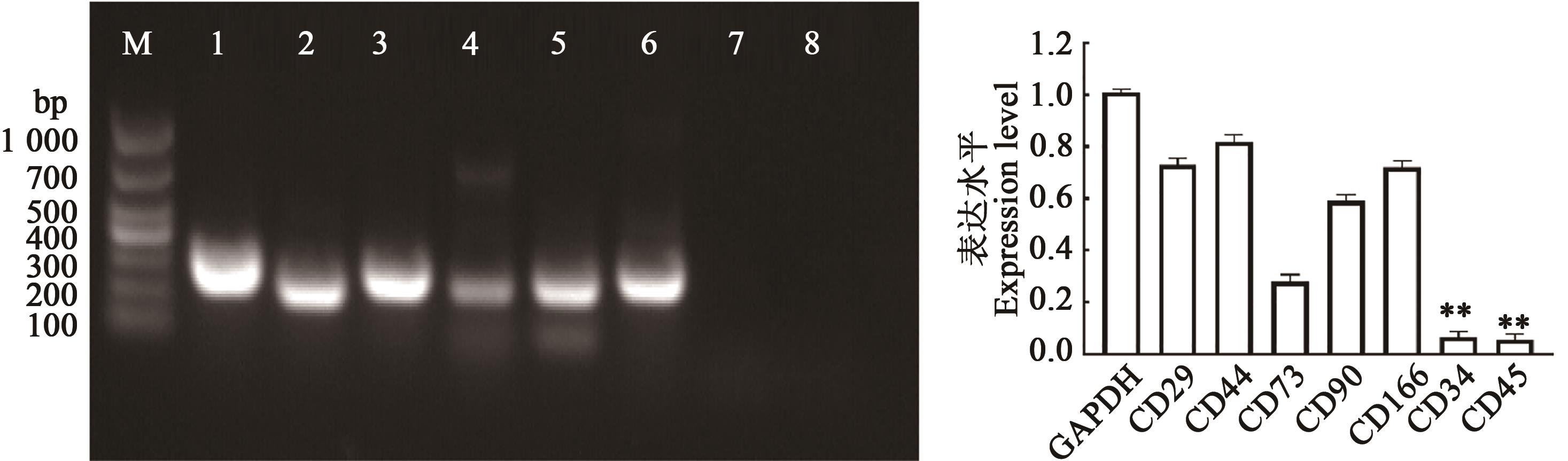
Fig. 5 RT-PCR result of marker genesNote: M shows DL1000 DNA Marker;1~8 show GAPDH, CD29, CD44, CD73, CD90, CD166, CD34, CD45 genes,respectively. ** indicates significant difference compared with GAPDH at P<0.01 level.
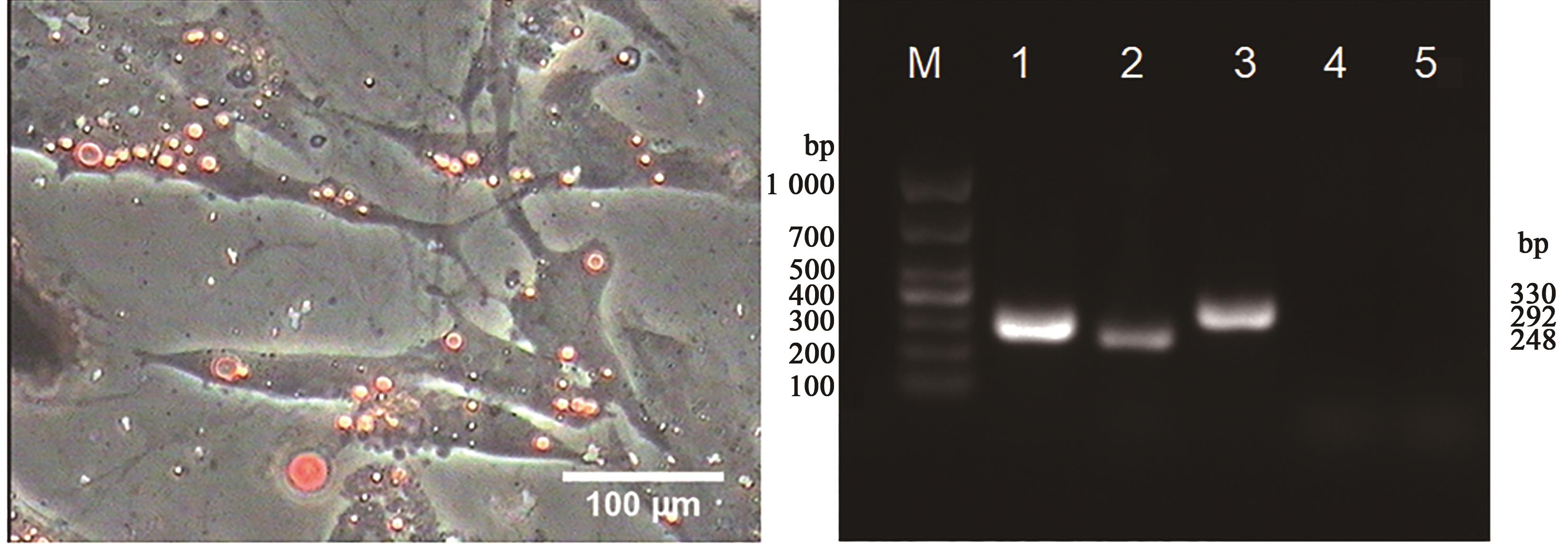
Fig. 6 Adipogenic differentiation of Gushi chicken UCMSCs tested by oilred O staining and RT-PCRNote: M shows DL1000 DNA Marker;1 shows GAPDH; 2~3 show LPL and PPAR-γ are positive in the inducted group; 4~5 show LPL and PPAR-γ in the control group.
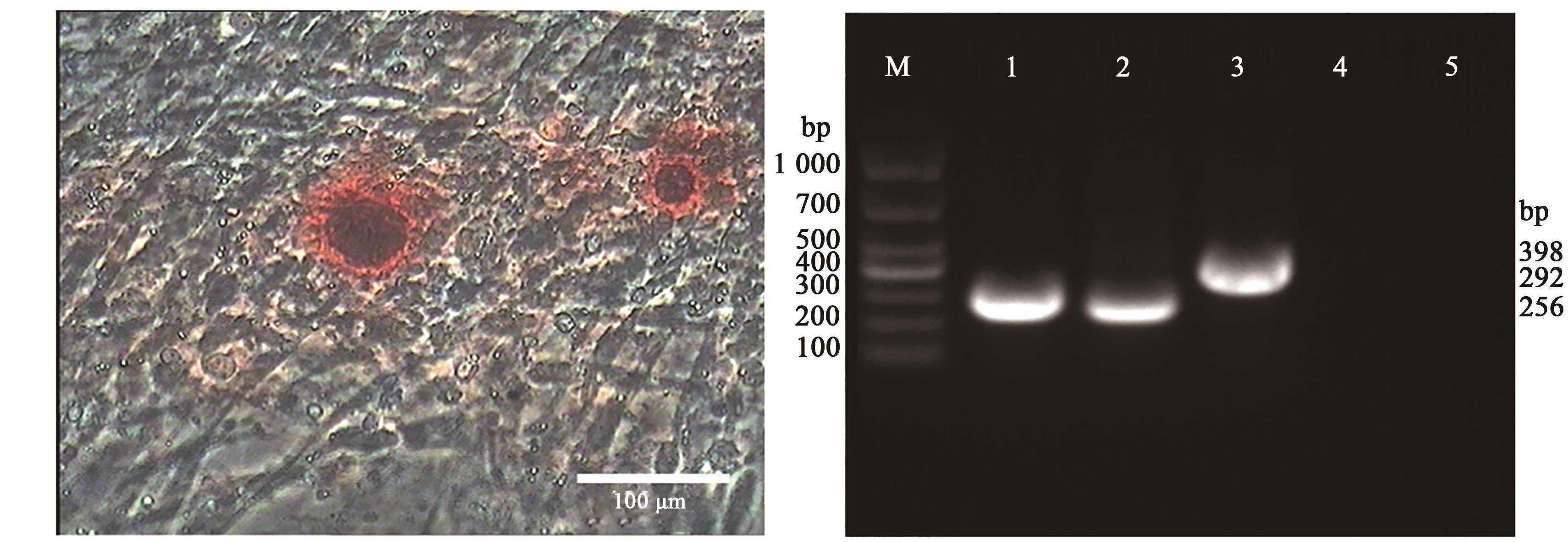
Fig. 7 Osteoblast differentiation of Gushi chicken UCMSCs by calcium deposits within differentiated osteoblast and RT-PCRNote: M shows DL1000 DNA Marker;1 shows GAPDH; 2~3 show collagen type I and osteopontin are positive in the inducted group; 4~5 show collagen type I and osteopontin are negative in the control group.

Fig. 8 Chondrocyte differentiation of Gushi chicken UCMSCs by allianz staining and RT-PCRNote: M shows DL1000 DNA Marker;1 shows GAPDH; 2~3 show SOX9 and ACAN are positive in the inducted group; 4~5 show SOX9 and ACAN are negative in the control group.
| 1 | PU L, MENG M, WU J, et al.. Compared to the amniotic membrane, Wharton’s jelly may be a more suitable source of mesenchymal stem cells for cardiovascular tissue engineering and clinical regeneration [J/OL]. Stem Cell Res. Ther.,2017, 8(1):72 [2022-03-19]. . |
| 2 | CHEN W, LIU X, CHEN Q, et al.. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs [J]. J. Tissue Eng. Regen. Med., 2018,12(1):191-203. |
| 3 | DZIERZAK E, BIGAS A. Blood development: hematopoietic stem cell dependence and independence [J]. Cell Stem Cell,2018,22(5):639-651. |
| 4 | ZHANG L, LI Y, GUAN C Y, et al.. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase [J/OL]. Stem Cell Res. Ther., 2018,9(1):36 [2022-03-19]. . |
| 5 | GOMES A, COELHO P, SOARES R, et al.. Human umbilical cord mesenchymal stem cells in type 2 diabetes mellitus: the emerging therapeutic approach [J]. Cell Tissue Res., 2021, 385(3):497-518. |
| 6 | STINER R, ALEXANDER M, LIU G, et al.. Transplantation of stem cells from umbilical cord blood as therapy for type Ⅰ diabetes [J]. Cell Tissue Res., 2019, 378(2):155-162. |
| 7 | WANG Y, LI H, LI X, et al.. Hypoxic Preconditioning of human umbilical cord mesenchymal stem cells is an effective strategy for treating acute lung injury [J]. Stem Cells Dev., 2021, 30(3):128-134. |
| 8 | SHU L, NIU C, LI R, et al.. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells [J/OL]. Stem Cell Res. Ther., 2020,11(1):361 [2022-03-19]. . |
| 9 | ZHONG Y, ZENG L, DENG J, et al.. β-hydroxy-β-methylbutyrate (HMB) improves mitochondrial function in myocytes through pathways involving PPARβ/δ and CDK4 [J]. Nutrition, 2019, 60:217-226. |
| 10 | LENG X, JIANG H. Effects of arachidonic acid and its major prostaglandin derivatives on bovine myoblast proliferation, differentiation, and fusion [J]. Domest. Anim. Endocrin., 2019, 67:28-36. |
| 11 | ZOU Y, WANG G, XU Y, et al.. Comparative study of the proliferative ability of skeletal muscle satellite cells under microwave irradiation in fractures with titanium alloy internal fixation in rabbits [J]. Exp Ther. Med., 2018, 16(6):4357-4366. |
| 12 | GEORGE S, HAMBLIN M R, ABRAHAMSE H. Differentiation of mesenchymal stem cells to neuroglia: in the context of cell signalling [J]. Stem Cell Rev. Rep., 2019, 15(6):814-826. |
| 13 | CAPLAN A I. Mesenchymal stem cells: time to change the name! [J] Stem Cells Transl. Med., 2017, 6(6):1445-1451. |
| 14 | ZHANG Y, WANG Y, LI Y, et al.. Genome-wide association study reveals the genetic determinism of growth traits in a Gushi-Anka F(2) chicken population [J]. Heredity, 2021, 126(2):293-307. |
| 15 | SHANG Y, GUAN H, ZHOU F. Biological characteristics of umbilical cord mesenchymal stem cells and its therapeutic potential for hematological disorders [J/OL]. Front. Cell Dev. Biol.,, 2021, 9:570179 [2022-03-19]. . |
| 16 | TORRE P, FLORES A I. Current status and future prospects of perinatal stem cells [J/OL]. Genes, 2020, 12(1):972313 [2022-03-19]. . |
| 17 | MUSHAHARY D, SPITTLER A, KASPER C, et al.. Isolation, cultivation, and characterization of human mesenchymal stem cells [J]. Cytometry Part A, 2018, 93(1):19-31. |
| 18 | CHEN J, PU Y, SUN Y, et al.. Biological characterization of metanephric mesenchymal stem cells from the Beijing duck [J]. Exp. Ther. Med., 2016, 11(2):439-447. |
| 19 | CHU D T, PHUONG T N T, TIEN N L B, et al.. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells [J/OL]. Int. J. Mol. Sci., 21 2020, 21(3):78 [2022-03-19]. . |
| 20 | DOMINICI M, LE BLANC K, MUELLER I, et al.. Minimal criteria for defining multipotent mesenchymal stromal cells [J].Cytotherapy, 2006, 8(4):315-317. |
| 21 | BEERAVOLU N, MCKEE C, ALAMRI A, et al.. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta [J/OL]. J. Visual. Exp., 2017(122):e55224 [2022-03-19]. . |
| 22 | LI K D, WANG Y, SUN Q, et al.. Rabbit umbilical cord mesenchymal stem cells: a new option for tissue engineering [J/OL]. J. Gene Med.,2021, 23(1):e3282 [2022-03-19]. . |
| 23 | GUILBAUD L, DUGAS A, WEBER M, et al.. In utero treatment of myelomeningocele with allogenic umbilical cord-derived mesenchymal stromal cells in an ovine model [J/OL]. Curr. Res. Transl. Med., 2022, 70(1):103314 [2022-03-19]. . |
| 24 | OTSUKA-YAMAGUCHI R, KITADA M, KURODA Y, et al.. Isolation and characterization of bone marrow-derived mesenchymal stem cells in Xenopus laevis [J/OL]. Stem Cell Res., 2021, 53:102341 [2022-03-19]. . |
| 25 | CIUFFREDA M C, MALPASSO G, MUSARÒ P, et al.. Protocols for in vitro differentiation of human mesenchymal stem cells into osteogenic, chondrogenic and adipogenic lineages [J]. Methods Mol. Biol., 2016, 1416:149-158. |
| 26 | CHEN S, TANG Y, LIU Y, et al.. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration [J/OL]. Cell Proliferat, 2019, 52(5):e12669 [2022-03-19]. . |
| 27 | LIU Q, LIN Z, LIU Y, et al.. Delivery of miRNA-29b using R9-LK15, a novel cell-penetrating peptide, promotes osteogenic differentiation of bone mesenchymal stem cells [J/OL]. BioMed. Res. Int., 2019, 2019:3032158 [2022-03-19]. . |
| 28 | LI Z, WANG P, LI J, et al.. The N(6)-methyladenosine demethylase ALKBH5 negatively regulates the osteogenic differentiation of mesenchymal stem cells through PRMT6 [J/OL]. Cell Death Dis.,2021, 12(6):578 [2022-03-19]. . |
| 29 | WANG S, ZHANG Y, XU Q, et al.. The differentiation of preadipocytes and gene expression related to adipogenesis in ducks (Anas platyrhynchos) [J/OL]. PLoS One, 2018, 13(5):e0196371 [2022-03-19]. . |
| 30 | FAYYAD A M, KHAN A A, ABDALLAH S H, et al.. Rosiglitazone enhances browning adipocytes in association with MAPK and PI3-K pathways during the differentiation of telomerase-transformed mesenchymal stromal cells into adipocytes [J/OL]. Int. J. Mol. Sci., 2019, 20(7):1618 [2022-03-19]. . |
| 31 | ZUBIRÍA M G, GIORDANO A P, GAMBARO S E, et al.. Dexamethasone primes adipocyte precursor cells for differentiation by enhancing adipogenic competency [J/OL]. Life Sci., 2020, 261:118363 [2022-03-19].. |
| 32 | LIN Z, HE H, WANG M, et al.. MicroRNA-130a controls bone marrow mesenchymal stem cell differentiation towards the osteoblastic and adipogenic fate [J/OL]. Cell Proliferat., 2019, 52(6):e12688 [2022-03-19]. . |
| 33 | YOU L, PAN L, CHEN L, et al.. MiR-27a is Essential for the shift from osteogenic differentiation to adipogenic differentiation of mesenchymal stem cells in postmenopausal osteoporosis [J]. Cell. Physiol. Biochem., 2016, 39(1):253-265. |
| 34 | SABEN J, THAKALI K M, LINDSEY F E, et al.. Distinct adipogenic differentiation phenotypes of human umbilical cord mesenchymal cells dependent on adipogenic conditions [J]. Exp. Biol. Med., 2014, 239(10):1340-1351. |
| 35 | LIU Y, LIN L, ZOU R, et al.. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis [J]. Cell Cycle,2018, 17(21-22):2411-2422. |
| 36 | PELTTARI K, STECK E, RICHTER W. The use of mesenchymal stem cells for chondrogenesis [J]. Injury, 2008,39():S58-S65. |
| 37 | LIN S, LEE W Y W, FENG Q, et al.. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation [J/OL]. Stem Cell Res Ther., 2017, 8(1):221 [2022-03-19].. |
| [1] | Zhong ZHUANG, Long ZHAO, Hao BAI, Yulin BI, Yingquan HUANG, Guohong CHEN, Guobin CHANG. Development of CRISPR/Cas9 and Its Application in Poultry [J]. Journal of Agricultural Science and Technology, 2022, 24(1): 14-23. |
| [2] | HUANG Jun, DING Hong-biao, ZHAO Guo-qi. Effect of Trehalose on the Growth and Carcass Performance of AA Broilers [J]. , 2009, 11(4): 58-63. |
| [3] | GU Xian-hong . Early Age Thermal Acclimation can Induce Thermotolerance |Acquisition in Broiler Challenged by Heat Stress [J]. , 2006, 8(1): 31-34. |
| [4] | . [J]. , 2003, 5(1): 64-66. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号