




















中国农业科技导报 ›› 2023, Vol. 25 ›› Issue (3): 21-29.DOI: 10.13304/j.nykjdb.2022.1091
收稿日期:2022-12-13
接受日期:2023-03-21
出版日期:2023-03-15
发布日期:2023-05-22
通讯作者:
柳小庆
作者简介:苗丽青 E-mail:miaolq27@163.com;
基金资助:
Liqing MIAO( ), Xuhui MA, Suzhen LI, Rumei CHEN, Xiaoqing LIU(
), Xuhui MA, Suzhen LI, Rumei CHEN, Xiaoqing LIU( )
)
Received:2022-12-13
Accepted:2023-03-21
Online:2023-03-15
Published:2023-05-22
Contact:
Xiaoqing LIU
摘要:
虾青素属于类胡萝卜素,具有较强的抗氧化活性及多种生物活性,因此被广泛应用于食品、化工、医疗等领域。虾青素存在立体异构和几何异构等多种异构体形式,不同异构体的生物活性存在差异,在商业化应用上也有不同。目前,商业化应用的虾青素主要来源于化学合成和微生物与雨生红球藻的提取物,但二者均存在一定的限制性因素。近年来,为了开辟新的虾青素生物合成途径,越来越多的研究报道了利用基因工程技术在植物中重构虾青素合成代谢途径合成虾青素。同时,通过提高虾青素的稳定性来拓展其应用范围,增强应用效果。对虾青素的特性、主要的生物合成途径及应用现状进行了综述,总结了有关虾青素合成的植物基因工程研究和活性递送系统的发展,并对虾青素的产业化应用进行了展望。
中图分类号:
苗丽青, 马旭辉, 李素贞, 陈茹梅, 柳小庆. 虾青素的生物合成与产业化应用[J]. 中国农业科技导报, 2023, 25(3): 21-29.
Liqing MIAO, Xuhui MA, Suzhen LI, Rumei CHEN, Xiaoqing LIU. Biosynthesis and Industrial Application of Astaxanthin[J]. Journal of Agricultural Science and Technology, 2023, 25(3): 21-29.

图1 虾青素分子主要的立体异构体和几何异构体A:左旋(3S,3’S)立体异构体;B:右旋(3R,3’R)立体异构体;C:内消旋(3R,3’S)立体异构体;D:全反式结构几何异构体;E:13-顺式结构几何异构体;F:9-顺式结构几何异构体
Fig. 1 Main stereoisomers and geometric isomers of astaxanthin moleculesA: Stereoisomers of levoisomer (3S, 3’S); B: Stereoisomers of dextroisomer (3R, 3’R); C: Stereoisomers of mesomer (3R, 3’S); D: Geometric isomer of all trans structures; E: Geometric isomer of 13-cis structures; F: Geometric isomer of 9-cis structures
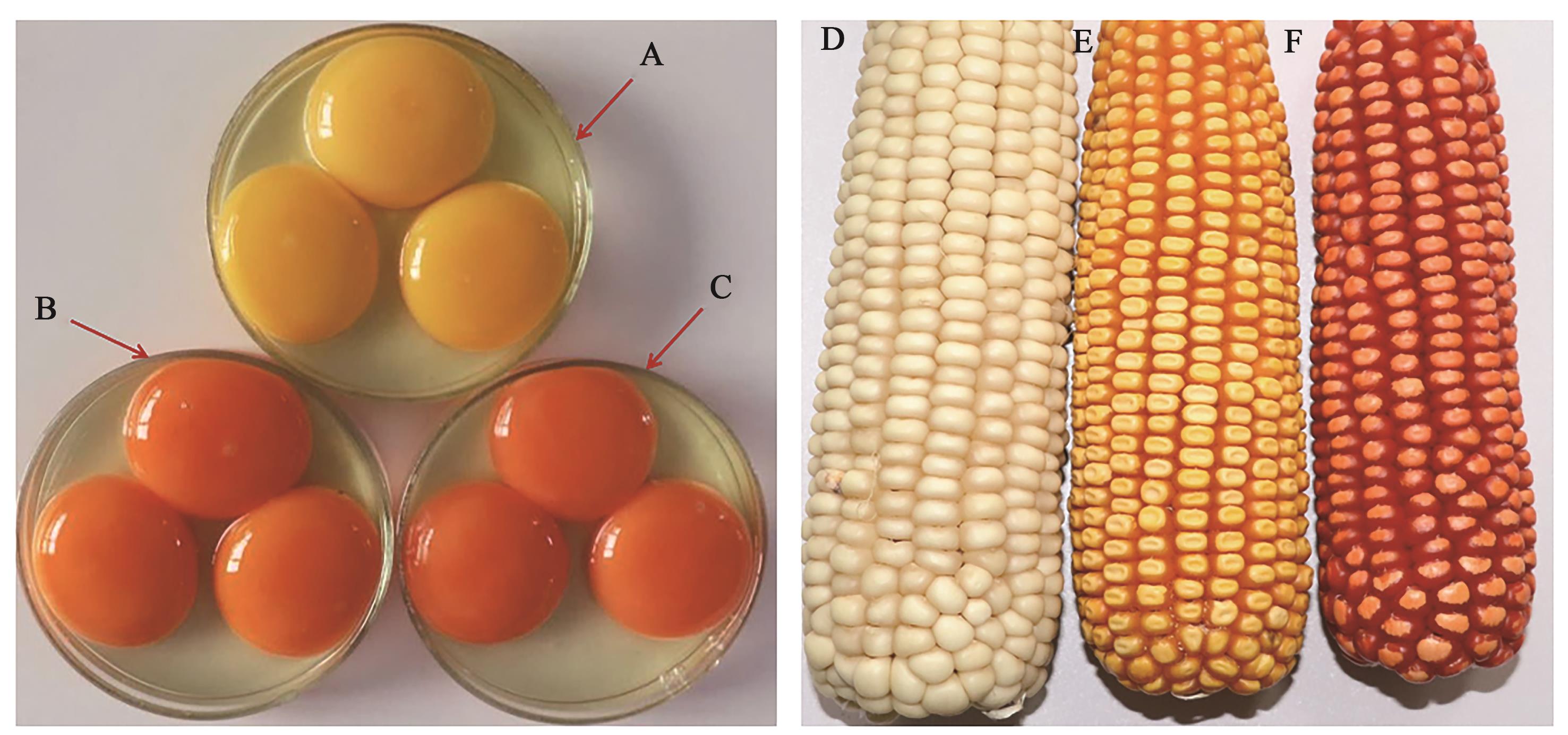
图3 高虾青素转基因玉米及其饲喂蛋鸡所产的鸡蛋A:饲喂普通饲料蛋鸡所产的鸡蛋;B:饲喂添加雨生红球藻藻粉饲料蛋鸡所产的鸡蛋;C:饲喂高虾青素玉米饲料蛋鸡所产的鸡蛋;D:白色玉米;E:黄色玉米;F高虾青素转基因玉米
Fig. 3 Astaxanthin corn and eggs from laying hensA: Eggs from hens fed with the common layer feed; B: Eggs from hens fed with the feed added Haematococcus pluvialis algae powder; C: Eggs from hens fed with high astaxanthin corn; D: White corn; E: Yellow corn; F: Transgenic maize with high level of astaxanthin
| 物种Species | 基因来源Source of gene | 年份Year | 含量Content |
|---|---|---|---|
红发夫酵母 Xanthophyllomyces dendrorhous (Phaffia rhodozyma) | — | 1999[ | 约干物质量的0.5% About 0.5% of dry weight |
| 红球藻Haematococcus pluvialis | — | 1999[ 2000[ | 干物质量的4%~5% 4%~5% of dry weight |
| 烟草Tobacco | Haematococcus pluvialis CrtO | 2000[ | 83.90 µg·g-1 FW |
| 马铃薯Potato | Pantoea ananatiscrtB Haematococcus pluvialis bkt1 | 2006[ | 13.90 μg·g-1 DW |
| 烟草Tobacco | Marine bacterium Brevundimonas sp., strain SD212 CrtW,CrtZ | 2008[ | 5.44 mg·g-1 DW |
| 胡萝卜Carrot | Haematococcus pluvialisBKT | 2008[ | 91.60 μg·g-1 FW |
| 拟南芥Arabidopsis thaliana | ChlamydomonasreinhardtiiBKT | 2011[ | 2.07 mg·g-1 DW |
| 烟草Tobacco | ChlamydomonasreinhardtiiBKT | 2012[ | 1.60 mg·g-1 DW |
| 番茄Tomato | ChlamydomonasreinhardtiiBKT Haematococcus pluvialis BHY | 2013[ | 16.10 mg·g-1 DW |
| 玉米Maize | Zea maysPSY1 Chlamydomonas reinhardtiiBKT Brevundimonas sp. Strain SD212 (MBIC 03018) CrtZ | 2016[ | 16.77 μg·g-1 DW |
| 水稻Rice | Zea maysPSY1 Pantoea ananatisCrtI Chlamydomonas reinhardtiiBKT Haematococcus pluvialis BHY | 2018[ | 16.23 μg·g-1 DW |
| 玉米Maize | Zea maysPSY1 Pantoea ananatisCrtI Chlamydomonas reinhardtiiBKT Haematococcus pluvialis BHY | 2021[ | 47.76~111.82 mg·kg-1 DW |
表 1 主要研究物种中的虾青素含量
Table 1 Contents of astaxanthin in main studied species
| 物种Species | 基因来源Source of gene | 年份Year | 含量Content |
|---|---|---|---|
红发夫酵母 Xanthophyllomyces dendrorhous (Phaffia rhodozyma) | — | 1999[ | 约干物质量的0.5% About 0.5% of dry weight |
| 红球藻Haematococcus pluvialis | — | 1999[ 2000[ | 干物质量的4%~5% 4%~5% of dry weight |
| 烟草Tobacco | Haematococcus pluvialis CrtO | 2000[ | 83.90 µg·g-1 FW |
| 马铃薯Potato | Pantoea ananatiscrtB Haematococcus pluvialis bkt1 | 2006[ | 13.90 μg·g-1 DW |
| 烟草Tobacco | Marine bacterium Brevundimonas sp., strain SD212 CrtW,CrtZ | 2008[ | 5.44 mg·g-1 DW |
| 胡萝卜Carrot | Haematococcus pluvialisBKT | 2008[ | 91.60 μg·g-1 FW |
| 拟南芥Arabidopsis thaliana | ChlamydomonasreinhardtiiBKT | 2011[ | 2.07 mg·g-1 DW |
| 烟草Tobacco | ChlamydomonasreinhardtiiBKT | 2012[ | 1.60 mg·g-1 DW |
| 番茄Tomato | ChlamydomonasreinhardtiiBKT Haematococcus pluvialis BHY | 2013[ | 16.10 mg·g-1 DW |
| 玉米Maize | Zea maysPSY1 Chlamydomonas reinhardtiiBKT Brevundimonas sp. Strain SD212 (MBIC 03018) CrtZ | 2016[ | 16.77 μg·g-1 DW |
| 水稻Rice | Zea maysPSY1 Pantoea ananatisCrtI Chlamydomonas reinhardtiiBKT Haematococcus pluvialis BHY | 2018[ | 16.23 μg·g-1 DW |
| 玉米Maize | Zea maysPSY1 Pantoea ananatisCrtI Chlamydomonas reinhardtiiBKT Haematococcus pluvialis BHY | 2021[ | 47.76~111.82 mg·kg-1 DW |
| 1 | KUHN R, SOERENSEN N. The coloring matters of the lobster (Astacus gammarus L.) [J]. Z Angew Chem., 1938, 51:465-466. |
| 2 | CUNNINGHAM F X, GANTT E. Elucidation of the pathway to astaxanthin in the flowers of Adonis aestivalis [J]. Plant Cell, 2011, 23(8):3055-3069. |
| 3 | MUSSAGY C U, PEREIRA J F B, DUFOSSÉ L, et al.. Advances and trends in biotechnological production of natural astaxanthin by Phaffia rhodozyma yeast [J/OL]. Critical Rev. Food Sci. Nutr., 2021:1968788 [2022-11-08].. |
| 4 | MULARCZYK M, MICHALAK I, MARYCZ K. Astaxanthin and other nutrients from Haematococcus pluvialis-multifunctional applications [J]. Marine Drugs, 2020, 18(9):459-468. |
| 5 | LI J, ZHU D, NIU J, et al.. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis [J]. Biotechnol. Adv., 2011, 29(6):568-74. |
| 6 | LIM K C, YUSOFF F M, SHARIFF M, et al.. Astaxanthin as feed supplement in aquatic animals [J]. Rev. Aquac., 2018, 10(3):738-773. |
| 7 | BAKER R T M, PFEIFFER A M, SCHÖNER F J, et al.. Pigmenting efficacy of astaxanthin and canthaxanthin in fresh-water reared Atlantic salmon, Salmo salar [J]. Animal Feed Sci. Technol., 2002, 99(1) 97-106. |
| 8 | HIRSCHBERG J. Carotenoid biosynthesis in flowering plants [J]. Curr. Opinion Plant Biol., 2001, 4(3):210-218. |
| 9 | CUNNINGHAM F X, GANTT E. A study in scarlet:enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis [J]. Plant J., 2005, 41(3):478-492. |
| 10 | KAJIWARA S, KAKIZONO T, SAITO T, et al.. Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli [J]. Plant Mol. Biol., 1995, 29(2):343-352. |
| 11 | SCHOEFS B T, N-ERMIKI, RACHADI J, et al.. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids [J]. FEBS Lett., 2001, 500(3):125-128. |
| 12 | OJIMA K, BREITENBACH J, VISSER H, et al.. Cloning of the astaxanthin synthase gene from Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and its assignment as a β-carotene 3-hydroxylase/4-ketolase [J]. Mol. Genet. Genomics, 2006, 275(2):148-158. |
| 13 | ÁLVAREZ V, RODRíGUEZ-SáIZ M, DE LA FUENTE J L, et al.. The crtS gene of Xanthophyllomyces dendrorhous encodes a novel cytochrome-P450 hydroxylase involved in the conversion of β-carotene into astaxanthin and other xanthophylls [J]. Fungal Genetics Biol., 2006, 43(4):261-272. |
| 14 | ALCAÍNO J, BARAHONA S, CARMONA M, et al.. Cloning of the cytochrome p450 reductase (crtR) gene and its involvement in the astaxanthin biosynthesis of Xanthophyllomyces dendrorhous [J/OL]. BMC Microbiol, 2008, 8:169 [2022-11-08]. . |
| 15 | CHOUBERT G, MILICUA J C G, GOMEZ R. The transport of astaxanthin in immature rainbow trout Oncorhynchus mykiss serum [J]. Comparative Biochem. Physiol. Part A: Physiol., 1994, 108(2):245-248. |
| 16 | HENMI H, HATA M, HATA M. Astaxanthin and/or canthaxanthin-actomyosin complex in salmon muscle [J]. Nippon Suisan Gakkaishi, 1989, 55(9):1583-1589. |
| 17 | MATTHEWS S, ROSS N, LALL S, et al.. Astaxanthin binding protein in Atlantic salmon [J]. Comparative Biochem. Physiol. Part B: Biochem. Mol. Biol., 2006, 144(2):206-214. |
| 18 | AMBATI R R, GOGISETTY D, ASWATHANARAYANA R G, et al.. Industrial potential of carotenoid pigments from microalgae:current trends and future prospects [J]. Critical Rev. Food Sci. Nutr., 2019, 59(12):1880-1902. |
| 19 | 李新杰,朱伟,姜威,等.天然虾青素对鸭肉品质和脂质氧化稳定性的影响[J].粮食与食品工业, 2012(6):43-45. |
| LI X J, ZHU W, JIANG W, et al.. Effect of natural astaxanthin on the quality and lipid oxidation stability of duck meat [J]. Cereal Feed Ind., 2012(6):43-45. | |
| 20 | LIU X, MA X, WANG H, et al.. Metabolic engineering of astaxanthin-rich maize and its use in the production of biofortified eggs [J]. Plant Biotechnol. J., 2021, 19(9):1812-1823. |
| 21 | RANGA RAO A, BASKARAN V, SARADA R, et al.. In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass - a repeated dose study [J]. Food Res. Int., 2013, 54(1):711-717. |
| 22 | MIKI W. Biological functions and activities of animal carotenoids [J]. Pure Appl. Chem., 1991, 63(1):141-156. |
| 23 | 国家市场监督管理总局.特殊食品信息查询平台[Z].[2022-11-08]. http://ypzsx.gsxt.gov.cn/specialfood/#/food. |
| 24 | 中国物品编码中心.中国商品信息服务平台[Z].[2022-11-08]. https://www.gds.org.cn/#/home/index. |
| 25 | OTA T. Prevention of NAFLD/NASH by astaxanthin and β-Cryptoxanthin [J/OL]. Adv. Exp. Med. Biol., 2021, 1261:21. [2022-11-08]. |
| 26 | FAKHRI S, ABBASZADEH F, DARGAHI L, et al.. Astaxanthin:a mechanistic review on its biological activities and health benefits [J]. Pharmacol. Res., 2018, 136(10):1-20. |
| 27 | LIGNELL A K E. Medicament for improvement of duration of muscle function or treatment of muscle disorders or diseases [P]. United States, US6245818. |
| 28 | 皮士卿,陈新志,胡四平,等.虾青素的合成[J].有机化学, 2007, 27(9):1126-1129. |
| PI S Q, CHEN X Z, HU S P, et al.. The synthesis of astaxanthin [J]. Chin. J. Organic Chem., 2007, 27(9):1126-1129. | |
| 29 | SOUKUP M, WIDMER E, LUKÁČ T. Technical procedures for the syntheses of Carotenoids and related compounds from 6-Oxo-isophorone: syntheses of (3R,3′R)-zeaxanthin. part Ⅱ [J]. Helvetica Chimica Acta, 1990, 73(4):868-873. |
| 30 | 陈丹,汪锋,蒋珊,等.虾青素化学和生物合成研究进展[J].食品工业科技, 2021, 42(21):445-453. |
| CHEN D, WANG F, JIANG S, et al.. Progress in the chemistry and biosynthesis of astaxanthin [J]. Sci. Technol. Food Ind., 2021, 42(21):445-453. | |
| 31 | BAUER A, MINCEVA M. Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid-liquid chromatography [J]. RSC Adv., 2019, 9(40):22779-22789. |
| 32 | SANDMANN G, ALBRECHT M, SCHNURR G, et al.. The biotechnological potential and design of novel carotenoids by gene combination in Escherichia coli [J]. Trends Biotechnol., 1999, 17(6):233-237. |
| 33 | BOUSSIBA S, BING W, YUAN J P, et al.. Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses [J]. Biotechnol. Letters, 1999, 21(7):601-604. |
| 34 | YUAN J P, CHEN F. Purification of trans-astaxanthin from a high-yielding astaxanthin ester-producing strain of the microalga Haematococcus pluvialis [J]. Food Chem., 2000, 68(4):443-448. |
| 35 | MANN V, HARKER M, PECKER I, et al.. Metabolic engineering of astaxanthin production in tobacco flowers [J]. Nat. Biotechnol., 2000, 18(8):888-892. |
| 36 | MORRIS W L, DUCREUX L J, FRASER P D, et al.. Engineering ketocarotenoid biosynthesis in potato tubers [J]. Metabolic Eng., 2006, 8(3):253-263. |
| 37 | HASUNUMA T, MIYAZAWA S, YOSHIMURA S, et al.. Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering [J]. Plant J., 2008, 55(5):857-868. |
| 38 | JAYARAJ J, DEVLIN R, PUNJA Z. Metabolic engineering of novel ketocarotenoid production in carrot plants [J]. Transgenic Res., 2008, 17(4):489-501. |
| 39 | ZHONG Y J, HUANG J C, LIU J, et al.. Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidopsis [J]. J. Exp. Bot., 2011, 62(10):3659-3669. |
| 40 | HUANG J, ZHONG Y, SANDMANN G, et al.. Cloning and selection of carotenoid ketolase genes for the engineering of high-yield astaxanthin in plants [J]. Planta, 2012, 236(2):691-699. |
| 41 | HUANG J C, ZHONG Y J, LIU J, et al.. Metabolic engineering of tomato for high-yield production of astaxanthin [J]. Metabolic Eng., 2013, 17:59-67. |
| 42 | FARRÉ G, PEREZ-FONS L, DECOURCELLE M, et al.. Metabolic engineering of astaxanthin biosynthesis in maize endosperm and characterization of a prototype high oil hybrid [J]. Transgenic Res., 2016, 25(4):477-849. |
| 43 | ZHU Q, ZENG D, YU S, et al.. From golden rice to aSTARice: bioengineering astaxanthin biosynthesis in rice endosperm [J]. Mol. Plant, 2018, 11(12):1440-1448. |
| 44 | ZHU C, GERJETS T, SANDMANN G. Nicotiana glauca engineered for the production of ketocarotenoids in flowers and leaves by expressing the cyanobacterial crtO ketolase gene [J]. Transgenic Res., 2007, 16(6):813-821. |
| 45 | RALLEY L, ENFISSI E M, MISAWA N, et al.. Metabolic engineering of ketocarotenoid formation in higher plants [J]. Plant J., 2004, 39(4):477-486. |
| 46 | KHALID N, SHU G, HOLLAND B J, et al.. Formulation and characterization of O/W nanoemulsions encapsulating high concentration of astaxanthin [J]. Food Res. Int., 2017, 102(12):364-371. |
| 47 | RIBEIRO H S, RICO L G, BADOLATO G G, et al.. Production of O/W emulsions containing astaxanthin by repeated premix membrane emulsification [J]. J. Food Sci., 2005, 70(2):117-123. |
| 48 | HAMA S, UENISHI S, YAMADA A, et al.. Scavenging of hydroxyl radicals in aqueous solution by astaxanthin encapsulated in liposomes [J]. Biol. Pharmaceutical Bull., 2012, 35(12):2238-2242. |
| 49 | PAN L, ZHANG S W, GU K R, et al.. Preparation of astaxanthin-loaded liposomes:characterization, storage stability and antioxidant activity [J]. CyTA-J. Food, 2018, 16(1):607-618. |
| 50 | HIGUERA-CIAPARA I, FELIX-VALENZUELA L, GOYCOOLEA F, et al.. Microencapsulation of astaxanthin in a chitosan matrix [J]. Carbohydrate Polymers, 2004, 56(1):41-45. |
| 51 | WANG Q, ZHAO Y Y, GUAN L, et al.. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability [J]. Food Chem., 2017, 227(7):9-15. |
| 52 | HU Q, HU S, FLEMING E, et al.. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity [J]. Int. J. Biol. Macromol., 2020, 151(5):747-756. |
| 53 | WANG T, HU Q, LEE J Y, et al.. Solid lipid-polymer hybrid nanoparticles by in situ conjugation for oral delivery of astaxanthin [J]. J. Agric. Food Chem., 2018, 66(36):9473-9480. |
| 54 | HUANG L, LI D, MA Y, et al.. Dietary fatty acid-mediated protein encapsulation simultaneously improving the water-solubility, storage stability, and oral absorption of astaxanthin [J/OL]. Food Hydrocolloids, 2022, 123(2):107152 [2022-11-08].. |
| 55 | EDELMAN R, ENGELBERG S, FAHOUM L, et al.. Potato protein-based carriers for enhancing bioavailability of astaxanthin [J]. Food Hydrocolloids, 2019, 96(11):72-80. |
| 56 | LEVINSON Y, ISRAELI-LEV G, LIVNEY Y. Soybean β-conglycinin nanoparticles for delivery of hydrophobic nutraceuticals [J]. Food Biophysics, 2014, 9(4):332-340. |
| [1] | 林敏, 王磊, 谷晓峰, 燕永亮, 刘柱, 涂涛, 姚斌. 农业基因回路设计合成技术发展动态与策略[J]. 中国农业科技导报, 2022, 24(12): 101-111. |
| [2] | 朱畇昊1,2,李璐1,赵乐1,2,董诚明1,2*. 地黄次生代谢产物生物合成基因表达水平与其含量的相关性分析[J]. 中国农业科技导报, 2018, 20(11): 36-43. |
| [3] | 雷彩燕,李静静,闫凤鸣*. 植物皂苷生物合成及调控研究进展[J]. , 2014, 16(4): 50-58. |
| [4] | 刘楠1,张晓燕2,周德庆1*. 虾青素纳米粒的制备及性能研究[J]. , 2013, 15(6): 35-39. |
| [5] | 宗元元,李博强,秦国政,张占全,田世平*. 棒曲霉素对果品质量安全的危害及其研究进展[J]. , 2013, 15(4): 36-41. |
| [6] | 张晓蓉 杨素萍. 光合细菌类胡萝卜素研究[J]. , 2005, 7(6): 26-30. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号