




















中国农业科技导报 ›› 2024, Vol. 26 ›› Issue (1): 125-132.DOI: 10.13304/j.nykjdb.2022.0736
• 动植物健康 • 上一篇
常晓宁1,2( ), 郭金英2, 荣成博1, 谷彤彤1, 刘宇1(
), 郭金英2, 荣成博1, 谷彤彤1, 刘宇1( )
)
收稿日期:2022-09-02
接受日期:2022-10-19
出版日期:2024-01-15
发布日期:2024-01-08
通讯作者:
刘宇
作者简介:常晓宁 E-mail:cnchang0421@163.com;
基金资助:
Xiaoning CHANG1,2( ), Jinying GUO2, Chengbo RONG1, Tongtong GU1, Yu LIU1(
), Jinying GUO2, Chengbo RONG1, Tongtong GU1, Yu LIU1( )
)
Received:2022-09-02
Accepted:2022-10-19
Online:2024-01-15
Published:2024-01-08
Contact:
Yu LIU
摘要:
北京欧文氏菌(Erwinia beijingensis)可引起刺芹侧耳细菌性软腐病,为明确该病原菌中糖基转移酶的功能,以糖基转移酶基因为研究对象,构建重组表达载体进行表达纯化;并测定蛋白活性,分析蛋白间相互作用。结果表明,成功构建了原核表达载体,获得4个可溶性蛋白。经亲和层析柱纯化获得MshA、WbnH2、EpsH及TuaG蛋白,活性测定显示4个蛋白均可以利用UDP-糖,并优先利用UDP-半乳糖。GST pull down证实WbnH2及TuaG蛋白分别与MshA及EpsH蛋白在体外具有相互作用。以上研究结果为进一步研究北京欧文氏菌糖基转移酶基因功能奠定了基础。
中图分类号:
常晓宁, 郭金英, 荣成博, 谷彤彤, 刘宇. 北京欧文氏菌4个糖基转移酶活性测定及蛋白相互作用分析[J]. 中国农业科技导报, 2024, 26(1): 125-132.
Xiaoning CHANG, Jinying GUO, Chengbo RONG, Tongtong GU, Yu LIU. Activity Determination of 4 Glycosyltransferases and Protein Interaction Analysis of Erwinia beijingensis[J]. Journal of Agricultural Science and Technology, 2024, 26(1): 125-132.
引物名称 Primer name | 引物序列 Primer sequence(5’-3’) | 产物长度 Product length/bp |
|---|---|---|
| mshA-F | GAAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGTCGAATAGTAACTTTGTTGTATGTTTGC | 1 058 |
| mshA-R | TCTCAGTGGTGGTGGTGGTGGTGCTCGAGGATGTTACCGCCCTCTAAAAGATACGATCTAAAATATTCTT | |
| wbnH1 F | GAAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGAGTAATGCCGGAAGGAAAATAATTTATAT | 1 044 |
| wbnH1 R | CAGCCGGATCTCAGTGGTGGTGGTGGTGGTGCTCGAGTAATGGAATCAACTCAAGATCTTTTTGGGCTTC | |
| wbnH2 F | CCCCGGGAATTTCCGGTGGTGGTGGTGGAATTCACTTGATCGTGATCCATAATGGTGTTCCTCCTCATGC | 642 |
| wbnH2 R | CGTCAGTCAGTCACGATGAATTAAGCTTGAGCTCGAGCTACCGAAGAAATATTTCCTGATACAATTTATTC | |
| EpsH F | AAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGAATGAGAAGATCGCATCCCCAAAACTCTCT | 936 |
| EpsH R | CAGTGGTGGTGGTGGTGGTGCTCGAGTTTTATAGTTAGCACTCTAGTTTTACGCAACAAAAAACGTAAAA | |
| tuaG F | CTGGAAGTTCTGTTCCAGGGGCCCCTGGGATCCATGAAAATAACAATAGTTACTGCTACATATAATTCTG | 685 |
| tuaG R | AGATCGTCAGTCAGTCACGATGCGGCCGCTCGAGTCATCCCCTTAGAATAGCTCTTAGAGCATAATTCAT | |
| His1422 F | AGAAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGACGCCTGTTTATAATAGAGCAGACTTAC | 732 |
| His1422 R | AGCCGGATCTCAGTGGTGGTGGTGGTGGTGCTCGAGCATCTTTCTAATTCCAGAACCGACTCTAATTATG | |
| GST1422 F | GGAAGTTCTGTTCCAGGGGCCCCTGGGATCCATGACGCCTGTTTATAATAGAGCAGACTTACTTAAAAAT | 714 |
| GST1422 R | TCGTCAGTCAGTCACGATGCGGCCGCTCGAGTTACATCTTTCTAATTCCAGAACCGACTCTAATTATGTT |
表1 试验引物
Table 1 Primers in this experiment
引物名称 Primer name | 引物序列 Primer sequence(5’-3’) | 产物长度 Product length/bp |
|---|---|---|
| mshA-F | GAAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGTCGAATAGTAACTTTGTTGTATGTTTGC | 1 058 |
| mshA-R | TCTCAGTGGTGGTGGTGGTGGTGCTCGAGGATGTTACCGCCCTCTAAAAGATACGATCTAAAATATTCTT | |
| wbnH1 F | GAAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGAGTAATGCCGGAAGGAAAATAATTTATAT | 1 044 |
| wbnH1 R | CAGCCGGATCTCAGTGGTGGTGGTGGTGGTGCTCGAGTAATGGAATCAACTCAAGATCTTTTTGGGCTTC | |
| wbnH2 F | CCCCGGGAATTTCCGGTGGTGGTGGTGGAATTCACTTGATCGTGATCCATAATGGTGTTCCTCCTCATGC | 642 |
| wbnH2 R | CGTCAGTCAGTCACGATGAATTAAGCTTGAGCTCGAGCTACCGAAGAAATATTTCCTGATACAATTTATTC | |
| EpsH F | AAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGAATGAGAAGATCGCATCCCCAAAACTCTCT | 936 |
| EpsH R | CAGTGGTGGTGGTGGTGGTGCTCGAGTTTTATAGTTAGCACTCTAGTTTTACGCAACAAAAAACGTAAAA | |
| tuaG F | CTGGAAGTTCTGTTCCAGGGGCCCCTGGGATCCATGAAAATAACAATAGTTACTGCTACATATAATTCTG | 685 |
| tuaG R | AGATCGTCAGTCAGTCACGATGCGGCCGCTCGAGTCATCCCCTTAGAATAGCTCTTAGAGCATAATTCAT | |
| His1422 F | AGAAATAATTTTGTTTAACTTTAAGAAGGAGATATACATATGACGCCTGTTTATAATAGAGCAGACTTAC | 732 |
| His1422 R | AGCCGGATCTCAGTGGTGGTGGTGGTGGTGCTCGAGCATCTTTCTAATTCCAGAACCGACTCTAATTATG | |
| GST1422 F | GGAAGTTCTGTTCCAGGGGCCCCTGGGATCCATGACGCCTGTTTATAATAGAGCAGACTTACTTAAAAAT | 714 |
| GST1422 R | TCGTCAGTCAGTCACGATGCGGCCGCTCGAGTTACATCTTTCTAATTCCAGAACCGACTCTAATTATGTT |
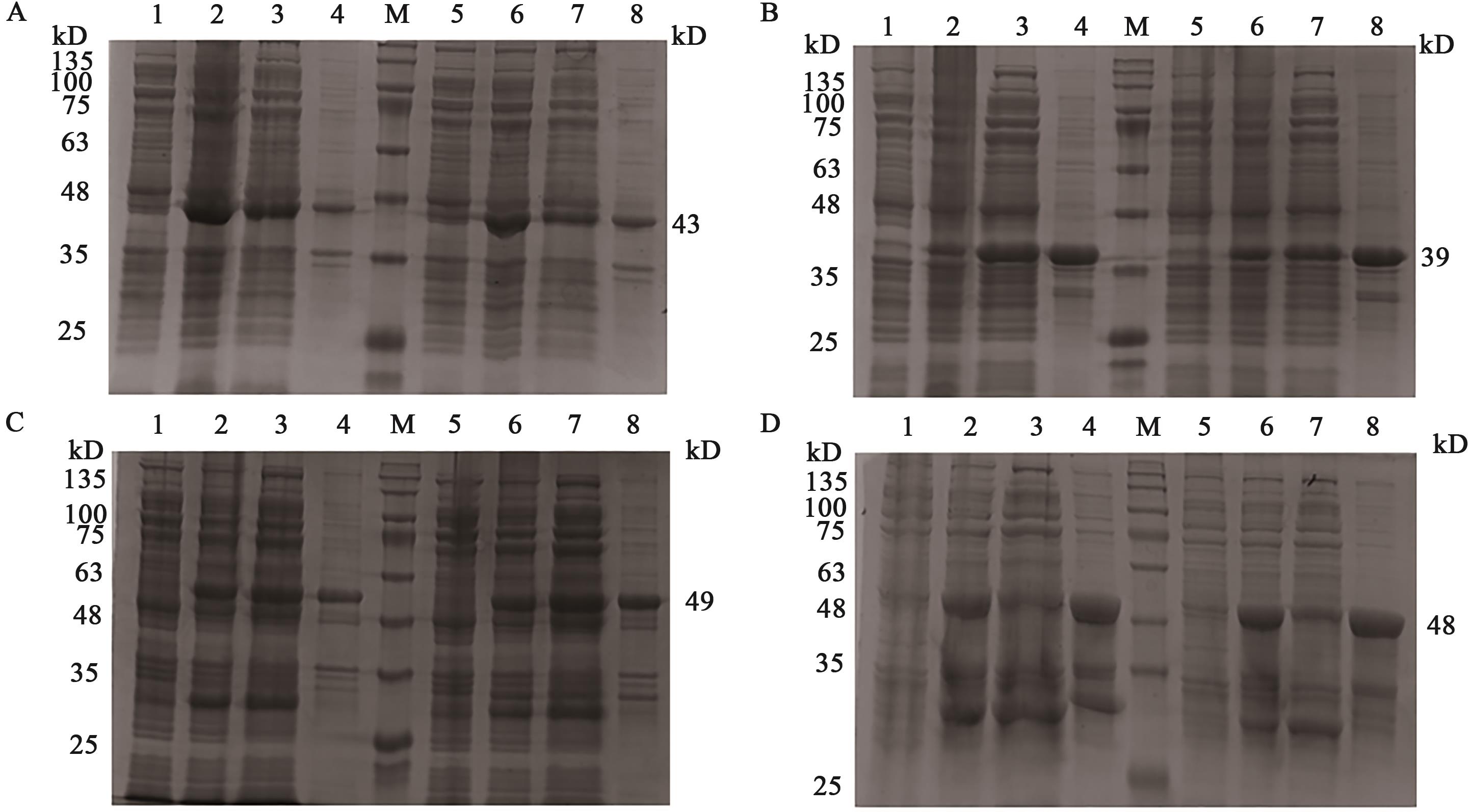
图1 小量诱导表达可溶性蛋白的筛选结果A:E. coli BL21/pET-30a-mshA;B:E. coli BL21/pET-30a-EpsH;C:E. coli BL21/pGEX-6p-tuaG;D:E. coli BL21/pGEX-wbnH2。1~4分别为0.1 mmol·L-1 IPTG诱导的诱导前全菌、诱导后全菌、诱导后破碎上清及诱导后破碎沉淀;M—marker;5~8分别为1.0 mmol·L-1 IPTG诱导的诱导前全菌、诱导后全菌、诱导后破碎上清及诱导后破碎沉淀
Fig. 1 Screening results of soluble protein induced by a small amount of induced expression.A: E. coli BL21/ pET-30a-mshA; B: E. coli BL21/pET-30a-EpsH;C: E. coli BL21/pGEX-6p-tuaG;D: E. coli BL21/pGEX-wbnH2. 1~4 are whole bacteria before induction, whole bacteria after induction, broken supernatant after induction and broken sediment after induction with 0.1 mmol·L-1 IPTG induced, respectively; M is marker; 5~8 are the whole bacteria before induction, whole bacteria after induction, broken supernatant after induction and broken sediment after induction induced with 1.0 mmol·L-1 IPTG, respectively
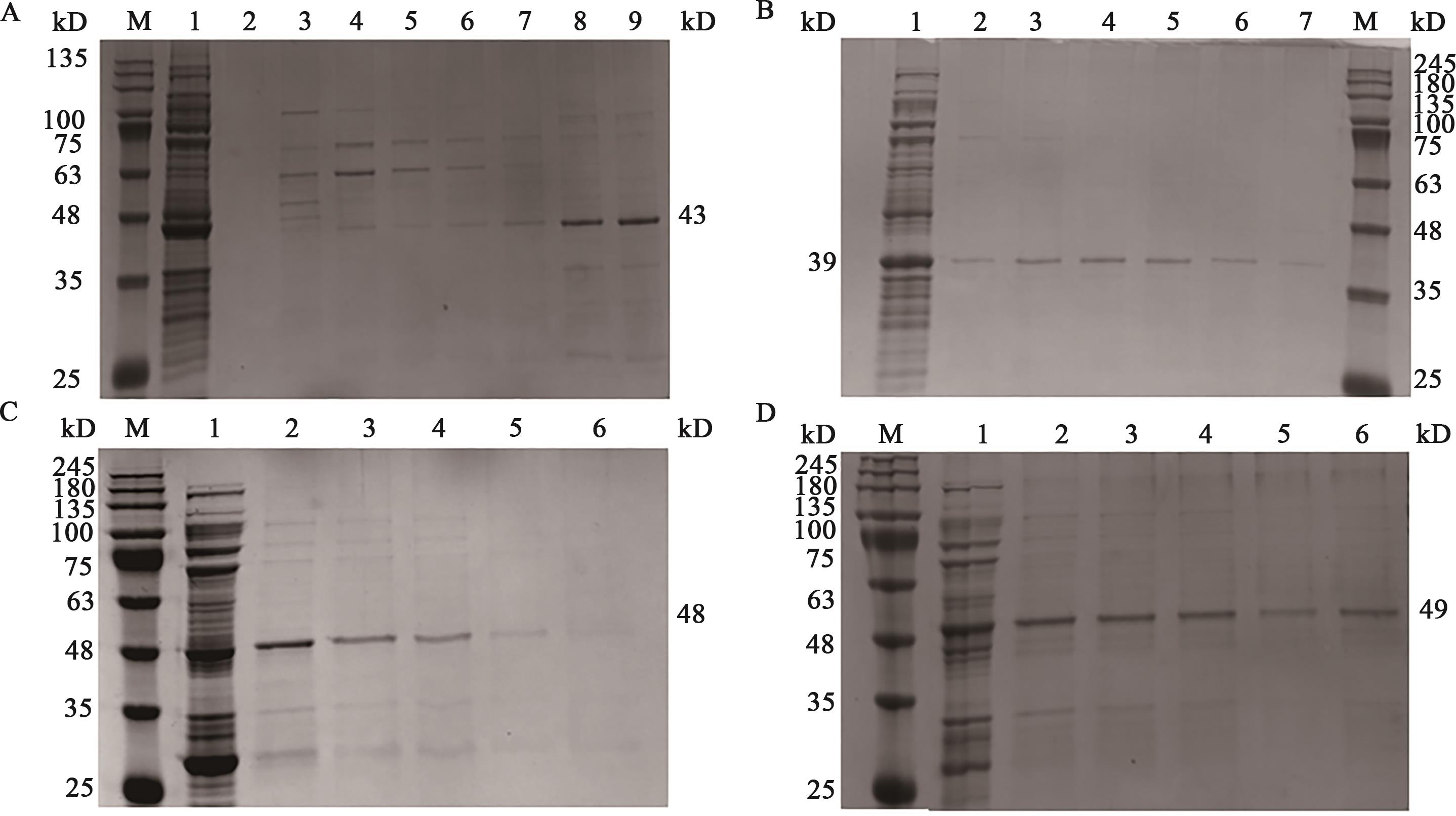
图2 蛋白纯化结果A:MshA蛋白纯化,其中M为marker;1为流穿液,2为缓冲液A洗脱,3~9分别为20、40、60、80、100、150、200 mmol·L-1咪唑浓度洗脱液;B:EpsH蛋白纯化,其中1为流穿液,2~6分别为60、80、100、150、200、300 mmol·L-1咪唑浓度洗脱液,M为marker;C:WbnH2蛋白纯化,其中M为marker,1为流穿液,2~6分别为20 mmol·L-1 GSH洗脱;D:TuaG蛋白纯化,其中M为marker,1为流穿液,2~4为10 mmol·L-1 GSH洗脱,5~6为10 mmol·L-1 GSH+5 mmol·L-1 DTT洗脱
Fig. 2 Protein purification resultsA: MshA protein purification, in which M is marker, 1 is running through liquid, 2 is buffer A elution, 3~9 are the eluents of 20, 40, 60, 80, 100, 150, 200 mmol L-1 imidazole concentration, respectively; B: EpsH protein purification, in which 1 is the flow through solution, 2~6 are 60, 80, 100, 150, 200, 300 mmol L-1 imidazole concentration eluates, respectively, M is marker; C: WbnH2 protein purification, in which M is marker, 1 is running through liquid, 2~6 are 20 mmol L-1 GSH elution; D: TuaG protein purification, in which M is marker, 1 is running through liquid, 2~4 is 10 mmol L-1 GSH elution, 5-6 is 10 mmol·L-1 GSH+5 mmol L-1 DTT elution
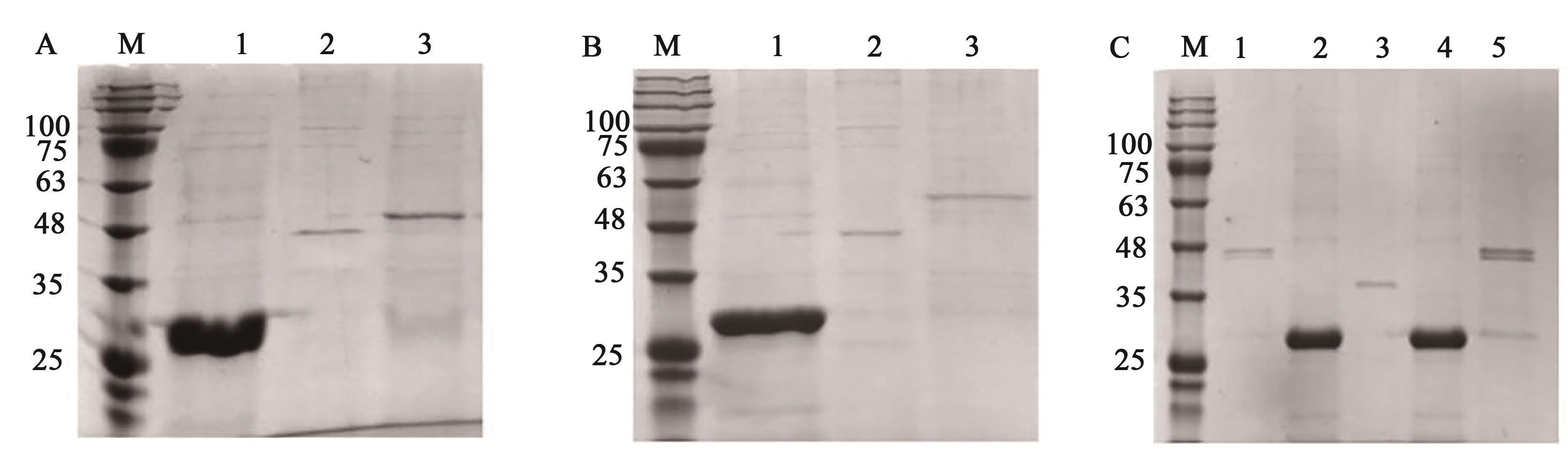
图4 GST pull down结果A:WbnH2与MshA蛋白的结合,M—Marker,1—GST与MshA蛋白结合,2—MshA蛋白,3—WbnH2与MshA蛋白结合;B:TuaG与MshA蛋白的结合,M—Marker,1—GST与MshA蛋白,2— MshA蛋白,3—TuaG与MshA蛋白结合;C:WbnH2、TuaG蛋白分别与EpsH蛋白结合,M—Marker,1—WbnH2与EpsH蛋白结合,2—GST与EpsH蛋白结合,3—EpsH蛋白,4—GST与EpsH蛋白结合,5—TuaG与EpsH蛋白结合
Fig. 4 GST pull down resultA: Binding between WbnH2 and MshA proteins, M—Marker, 1—GST binds to MshA protein, 2—MshA protein, 3—WbnH2 binds to MshA protein; B: Binding between TuaG and MshA proteins, M—Marker, 1—GST binds to MshA proteins, 2—MshA protein, 3—TuaG binds to MshA protein; C: Binding between WbnH2 and TuaG proteins to EpsH protein, respectively, M—Marker, 1—WbnH2 binds to EpsH protein, 2—GST binds to EpsH protein, 3—EpsH protein, 4—GST binds to EpsH protein, 5—TuaG binds to EpsH protein
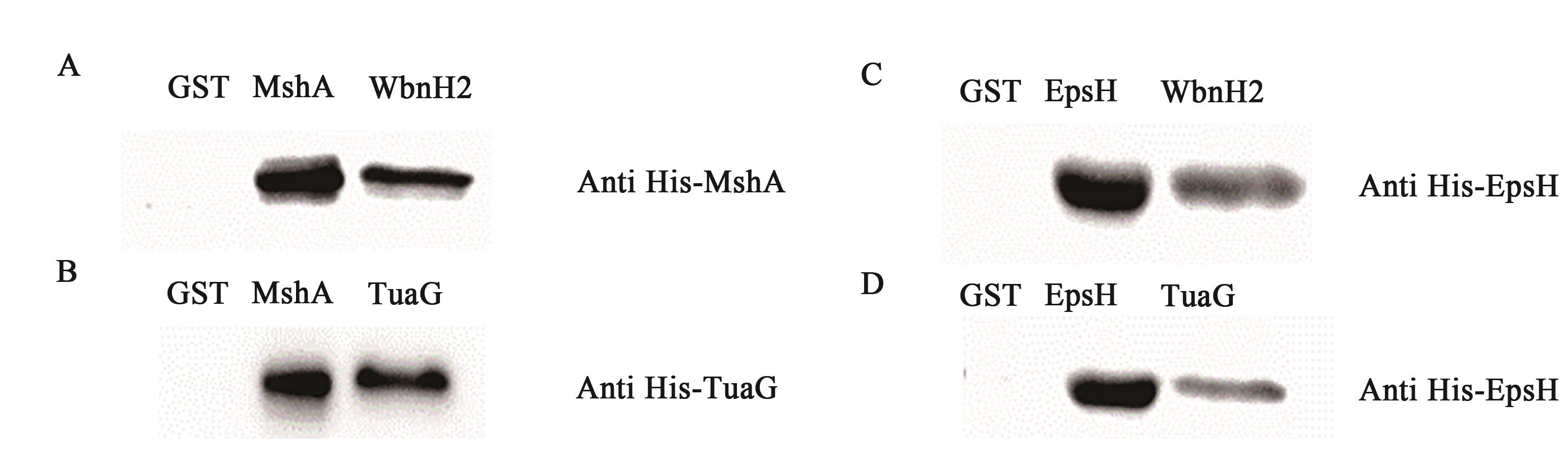
图5 Western-blot 检测结果A:WbnH2与MshA蛋白结合,1—GST与MshA蛋白结合,2—MshA蛋白,3—WbnH2与MshA蛋白结合;B:TuaG与MshA蛋白结合,1—GST与MshA蛋白结合,2—MshA蛋白,3—TuaG与MshA蛋白;C:WbnH2与EpsH蛋白结合,1—GST与EpsH蛋白结合;2—EpsH蛋白;3—WbnH2与EpsH蛋白结合;D:TuaG与EpsH蛋白结合,1—GST与EpsH蛋白结合;2—EpsH蛋白;3—TuaG与MshA蛋白结合
Fig. 5 Western blot test resultA: Binding between WbnH2 and MshA proteins. 1—GST binds to MshA protein; 2—MshA protein; 3—WbnH2 binds to MshA protein; B: Binding between TuaG and MshA proteins. 1—GST binds to MshA protein; 2—MshA protein; 3—TuaG binds to MshA protein; C: Binding between WbnH2 and EpsH proteins, 1—GST binds to EpsH protein; 2—EpsH protein; 3—WbnH2 binds to EpsH protQein; D: Binding between TuaG and EpsH proteins, 1—GST binds to EpsH protein, 2—EpsH protein; 3—TuaG binds to MshA protein
| 1 | WANG Q, ZHANG C, WU X, et al.. Chitosan augments tetramycin against soft rot in kiwifruit and enhances its improvement for kiwifruit growth, quality and aroma [J]. Biomolecules, 2021, 11(9):1257-1270. |
| 2 | LIU M, WU F, WANG S, et al.. Comparative transcriptome analysis reveals defense responses against soft rot in Chinese cabbage [J]. Hortic. Res., 2019, 6:68-86. |
| 3 | SUN M, LIU H, HUANG J, et al.. A loop-mediated isothermal amplification assay for rapid detection of Pectobacterium aroidearum that causes soft rot in konjac [J]. Int. J. Mol. Sci., 2019, 20(8):1937-1950. |
| 4 | YAKOVLIEVA L, WALVOORT M. Processivity in bacterial glycosyltransferases [J]. ACS Chem. Biol., 2019, 15(1):3-16. |
| 5 | KIM M K, LEE S M, SEUK S W, et al.. Characterization of the rcsA gene from Pantoea sp. strain PPE7 and its influence on extracellular polysaccharide production and virulence on Pleurotus eryngii [J]. Plant Pathol. J., 2017, 33(3):276-287. |
| 6 | XU F, YAN H, LIU Y, et al.. A re-evaluation of the taxonomy and classification of the type Ⅲ secretion system in a pathogenic bacterium causingsoft rot disease of Pleurotus eryngii [J]. Curr. Microbiol., 2021, 78(1):179-189. |
| 7 | 赖亮民,胡晶晶,王艳,等.刺芹侧耳软腐病病原菌鉴定及其致病机制[J].食用菌学报,2021,28(2):89-99. |
| LAI L M, HU J J, WANG Y, et al.. Identification and pathogenic mechanisms of pathogens causing soft rot disease in Pleurotus eryngii [J]. Acta Edulis Fungi, 2021, 28(2):89-99. | |
| 8 | 张瑞颖,胡丹丹,顾金刚,等.刺芹侧耳细菌性软腐病病原菌分离鉴定[J].食用菌学报,2013,20(3):43-49. |
| ZHANG R Y, HU D D, GU J G, et al.. Identification and characterization of an Erwinia sp. causing bacterial soft-rot disease on pleurotuseryngii cultivated in China [J]. Acta Edulis Fungi, 2013, 20(3):43-49. | |
| 9 | 马元伟,马康,王守现,等.一株刺芹侧耳软腐病致病菌新种的分离与鉴定[C]//第十届全国食用菌学术研讨会论文汇编,北京,2014:382-389. |
| MA Y M, MA K, WANG S X, et al.. Isolation and identification of a novel pathogenic bacteria,causing soft-rot diease of Pleurotus eryngii [C]// Proceedings of the 10th National Symposium on edible fungi, Beijing, 2014:382-389. | |
| 10 | LERMINIAUX N A, MACKENZIE K D, CAMERON A. Salmonella pathogenicity island 1 (SPI-1): the evolution and stabilization of a core genomic type three secretion system [J]. Microorganisms, 2020, 8(4):2-22. |
| 11 | KLEE S M, SINN J P, CHRISTIAN E, et al.. Virulence genetics of an Erwinia amylovora putative polysaccharide transporter family member [J]. J. Bacteriol., 2020, 202(22):1-19. |
| 12 | ROBERTS, IAN S. The biochemistry and genetics of capsular polysaccharide production in bacteria [J]. Annu. Rev. Microbiol., 1996, 50(1):285-315. |
| 13 | ASKARIAN F, UCHIYAMA S, MASSON H, et al.. The lytic polysaccharide monooxygenase CbpD promotes Pseudomonas aeruginosa virulence in systemic infection [J]. Nat. Comm., 2021, 12(1):1-19. |
| 14 | BAE N, PARK H J, PARK H, et al.. Deciphering the functions of the outer membrane porin OprBXo involved in virulence, motility, exopolysaccharide production, biofilm formation and stress tolerance in Xanthomonas oryzae pv. Oryzae [J]. Mol. Plant Pathol., 2018, 19(12):2527-2542. |
| 15 | JEON J G, ROSALEN P L, FALSETTA M L, et al.. Natural products in caries research: current (limited) knowledge, challenges and future perspective [J]. Caries Res., 2011, 45(3):243-263. |
| 16 | BISWAS A, THATTAI M. Promiscuity and specificity of eukaryotic glycosyltransferases [J]. Portland Press Open Access., 2020, 48(3):891-900. |
| 17 | LU Q, LI S, SHAO F. Sweet talk: protein glycosylation in bacterial interaction with the host [J]. Trends Microbiol., 2015, 23(10):630-641. |
| 18 | MIDDLETON D R, ACEIL J, MUSTAFA S, et al.. Glycosyltransferases within the psrP locus facilitate pneumococcal virulence [J]. J. Bacteriol., 2021, 203(7):389-400. |
| 19 | JIANG Y L, JIN H, YANG H B, et al.. Defining the enzymatic pathway for polymorphic O-glycosylation of the pneumococcal serine-rich repeat protein PsrP [J]. J. Biol. Chem., 2017, 292(15):6213-6224.. |
| 20 | DENG Q, WU H, GU Q, et al.. Glycosyltransferase FvCpsA regulates fumonisin biosynthesis and virulence in Fusarium verticillioides [J]. Toxins, 2021, 13(10):718-730. |
| 21 | ECHEVERZ M, GARCÍA B, SABALZA A, et al.. Lack of the PGA exopolysaccharide in Salmonella as an adaptive trait for survival in the host [J]. PLoS Genetics, 2017, 13(5):816-843. |
| 22 | EMI L, TYKESSO N, YAN G, et al.. Deciphering the mode of Action of the processive polysaccharide modifying enzyme dermatan sulfate epimerase 1 by hydrogen-deuterium exchange mass spectrometry [J]. Chem. Sci., 2016, 7(2):1447-1456. |
| 23 | DENG S, SUN W, DONG L, et al.. MoGT2 is essential for morphogenesis and pathogenicity of Magnaporthe oryzae [J]. Msphere, 2019, 4(5):309-319. |
| 24 | PÉREZ-PASCUAL D, GÓMEZ E, GUIJARRO J A. Lack of a type-2 glycosyltransferase in the fish pathogen Flavobacterium psychrophilum determines pleiotropic changes and loss of virulence [J]. Veterinary Res., 2015, 46(1):1-9. |
| 25 | DABRAL N, JAIN-GUPTA N, SELEEM M N, et al.. Overexpression of Brucella putative glycosyltransferase WbkA in B. abortus RB51 leads to production of exopolysaccharide [J]. Front. Cellular Infect. Microbiol., 2015, 5(4):54-68. |
| 26 | SUSAN G, KATHERINE, et al.. Structural determinants of the interaction between the TpsA and TpsB proteins in the Haemophilus influenzae HMW1 two-partner secretion system [J]. J. Bacteriol., 2015, 197(10):1769-1781. |
| 27 | ECHLIN H, ZHU F, LI Y, et al.. Gap2 promotes the formation of a stable protein complex required for mature fap1 biogenesis [J]. J. Bacteriol., 2013, 195(10):2166-2176. |
| [1] | 隋傅1,2,刘晓琳1,2,解志红1,3*. 茎瘤固氮根瘤菌ORS571受体TlpA1对琥珀酸的感应机理[J]. 中国农业科技导报, 2020, 22(10): 77-84. |
| [2] | 于晓庆1,谢华1,王秀玲2,马荣才1. 植物软腐病防御反应分子机制研究进展[J]. , 2012, 14(6): 49-53. |
| [3] | 崔严方,孙佩龙,刘新奇. EIAV gp45和 HIV gp120基因在不同表达系统中表达效果比较[J]. , 2011, 13(4): 72-78. |
| [4] | 王建设1,2,柏映国2,尹俊1,姚斌2. 脂环酸芽孢杆菌Alicyclobacillus sp. A15木聚糖酶基因xynA15的克隆表达及性质研究[J]. , 2010, 12(6): 114-119. |
| [5] | 刘宇1,2,闫彩霞1,张廷婷1,李春娟1,郑奕雄3,单世华1. 花生NBS-LRR类抗病基因的克隆及原核表达[J]. , 2010, 12(3): 73-78. |
| [6] | 李蕊沁1,冯树丹1,于莹2,吕召志1,黎莉1,徐明华1,尹悦佳3,郝东云3. 羊草几丁质酶ClassⅡ基因的克隆、生物信息学分析及原核表达[J]. , 2010, 12(2): 103-110. |
| [7] | 姚国玉,李宁,石鹏君,陈强,姚斌. 链霉菌Streptomyces sp. S9木聚糖酶基因xynBS9的克隆表达及性质分析[J]. , 2009, 11(4): 64-70. |
| [8] | 刘权,李广悦,曾洪梅,杨秀芬,邱德文. 微生物蛋白激发子PeaT1的获得及诱导水稻抗旱性的初步研究[J]. , 2009, 11(3): 51-55. |
| [9] | 段文娟,杨娇艳,李卓夫,张伟,张志芳. 来源于嗜热古细菌Pyrococcus furiosus乳糖酶基因的原核表达及酶学性质分析[J]. , 2008, 10(2): 76-81. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号