




















中国农业科技导报 ›› 2024, Vol. 26 ›› Issue (9): 54-61.DOI: 10.13304/j.nykjdb.2023.0176
收稿日期:2023-03-13
接受日期:2023-11-22
出版日期:2024-09-15
发布日期:2024-09-13
作者简介:李丹E-mail: lidans10@126.com
基金资助:Received:2023-03-13
Accepted:2023-11-22
Online:2024-09-15
Published:2024-09-13
摘要:
为提高以前体形式合成的蛋白质折叠与成熟效率,以枯草杆菌蛋白酶SprD为研究对象,通过蛋白重组表达、氨基酸定点突变、包涵体纯化结合体外复性、酶催化性能测定等方法比较各前体蛋白折叠成熟情况。结果表明,野生型前导肽介导的SprD前体蛋白可以在230 min内完成成熟,时间较长;成熟肽序列中E112A、S221A及S221C等突变导致相应前体蛋白成熟时间延长或成熟失败;而前导肽串联表达和切割位点酪氨酸缺失等措施可以将蛋白成熟时间缩短至80~160 min,成熟效率提高0.5~2.0倍,且成熟酶催化能力不受影响。前导肽序列中单个氨基酸位点改变对蛋白成熟影响较小,但对酶催化能力提升有一定帮助。因此,前导肽介导的蛋白成熟与酶催化能力的改变是2个相对独立的过程,前导肽表达方式与切割位点变化是促进蛋白体外成熟的较优方案。研究结果为加速蛋白成熟与蛋白改造提供了可参考方法。
中图分类号:
李丹. 前导肽介导的SprD蛋白体外折叠研究[J]. 中国农业科技导报, 2024, 26(9): 54-61.
Dan LI. Study on Folding of SprD Protein Mediated by Pro-peptide in vitro[J]. Journal of Agricultural Science and Technology, 2024, 26(9): 54-61.
引物名称 Primer name | 引物序列 Primer sequence (5’ - 3’) |
|---|---|
| pro-sprD sense | |
| pro-sprD anti | |
| Y-1D’ sense | GATCACGTTGCACAAGCGGCGCAGTCCGTGCCTTAC |
| Y-1D’ anti | GGCACGGACTGCGCCGCTTGTGCAACGTGATC |
| G-44D sense | GTCATTTCTGAAAAAGACGGGAAAGTGCAAAAGC |
| G-44D anti | GCTTTTGCACTTTCCCGTCTTTTTCAGAAATGAC |
| I-48V sense | CCAAGAAAAAAGATGTC GTTTCTGAAAAAGGCG |
| I-48V anti | CGCCTTTTTCAGAAAC GACATCTTTTTTCTTGG |
| E112A sense | CGGAATTGCGTGGGCGATCGCAAACAATATC |
| E112A anti | GCGATCGCCCACGCAATTCCGTTAATGATC |
| S221A sense | GTACAATGGTACG GCAATGGCATCTCCGCACG |
| S221A anti | GCGGAGATGC CATTGCCGTACCATTGTACG |
| S221C sense | GTGCGGAGATGCCAT ACACGTACCATTGTACGCGC |
| S221C anti | GTACAATGGTACGTGT ATGGCATCTCCGCACGT |
表1 研究所用引物
Table 1 Primers used in the study
引物名称 Primer name | 引物序列 Primer sequence (5’ - 3’) |
|---|---|
| pro-sprD sense | |
| pro-sprD anti | |
| Y-1D’ sense | GATCACGTTGCACAAGCGGCGCAGTCCGTGCCTTAC |
| Y-1D’ anti | GGCACGGACTGCGCCGCTTGTGCAACGTGATC |
| G-44D sense | GTCATTTCTGAAAAAGACGGGAAAGTGCAAAAGC |
| G-44D anti | GCTTTTGCACTTTCCCGTCTTTTTCAGAAATGAC |
| I-48V sense | CCAAGAAAAAAGATGTC GTTTCTGAAAAAGGCG |
| I-48V anti | CGCCTTTTTCAGAAAC GACATCTTTTTTCTTGG |
| E112A sense | CGGAATTGCGTGGGCGATCGCAAACAATATC |
| E112A anti | GCGATCGCCCACGCAATTCCGTTAATGATC |
| S221A sense | GTACAATGGTACG GCAATGGCATCTCCGCACG |
| S221A anti | GCGGAGATGC CATTGCCGTACCATTGTACG |
| S221C sense | GTGCGGAGATGCCAT ACACGTACCATTGTACGCGC |
| S221C anti | GTACAATGGTACGTGT ATGGCATCTCCGCACGT |
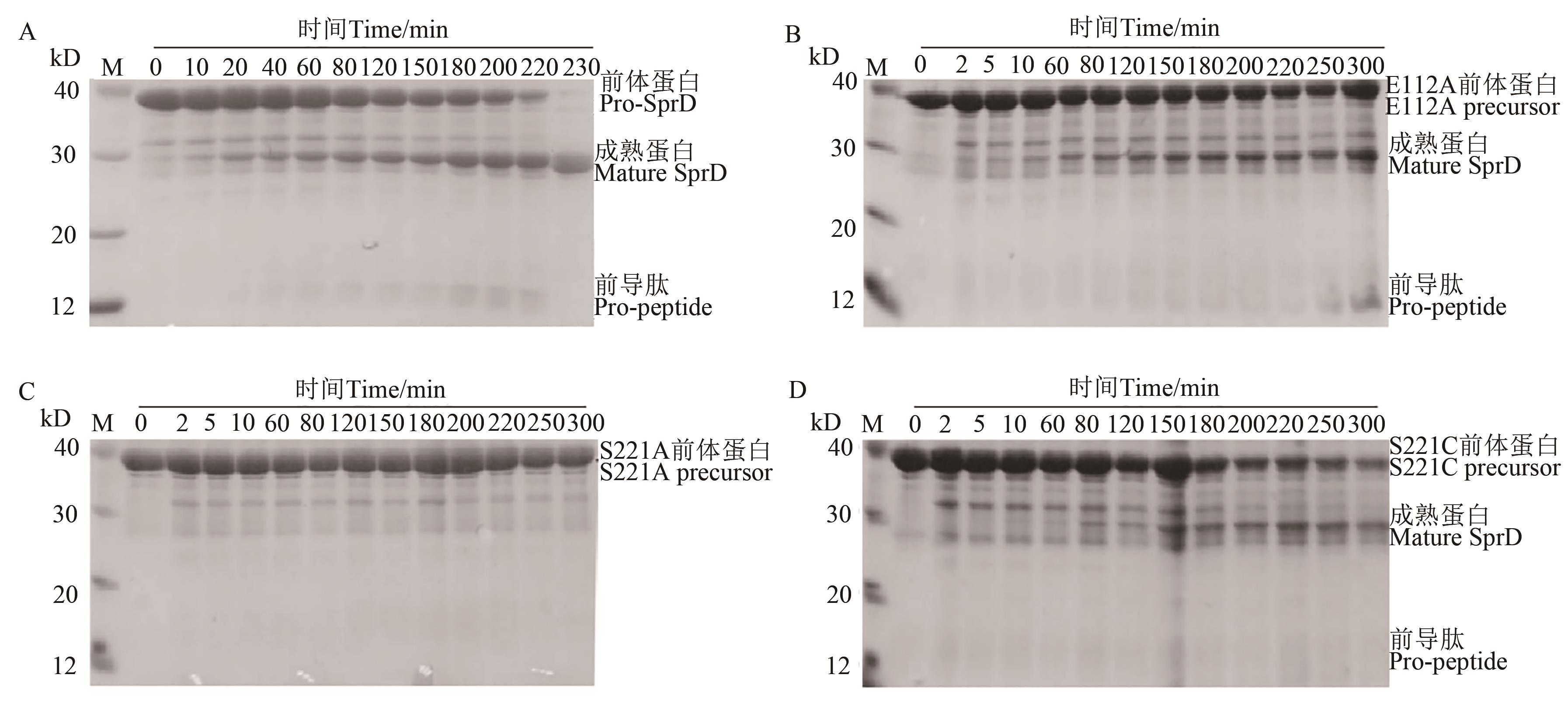
图2 野生型前体蛋白体外加工为成熟酶A: 野生型前体蛋白的折叠与成熟;B~D: E112A、S221A和S221C前体蛋白的折叠与成熟
Fig. 2 Processing of wild-type precursors to mature enzymes in vitroA: Folding and maturation of wild-type precursor; B~D: Folding and maturation of E112A, S221A and S221C precursors
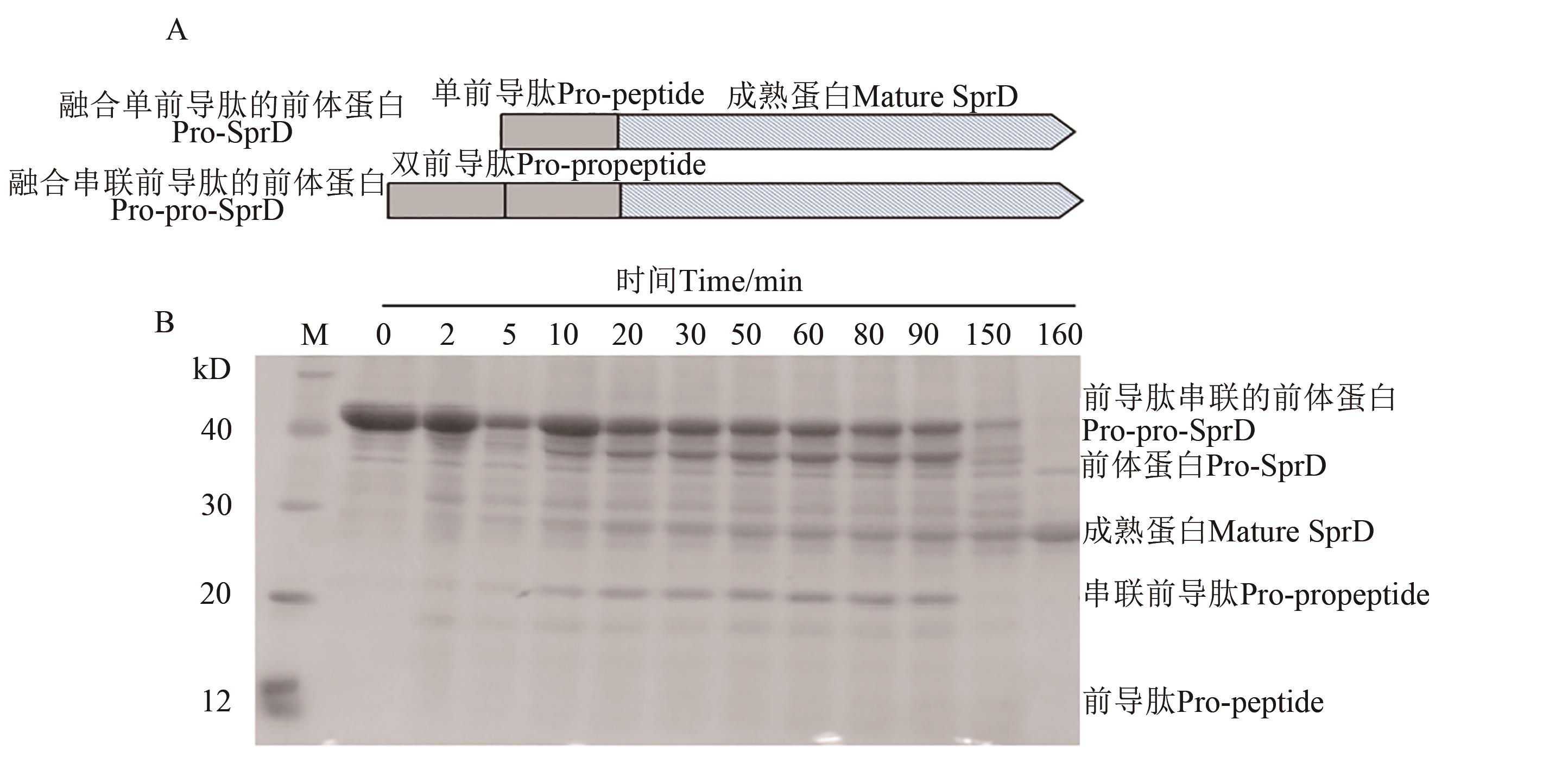
图3 Pro-pro-SprD前体蛋白的成熟过程A:前导肽串联表达示意图;B: Pro-pro-SprD前体蛋白的加工与成熟
Fig. 3 Maturation process of pro-pro-SprD precursorA: Schematic of tandem expression of pro-peptides; B: Processing and maturation of pro-pro-SprD precursors
| 前体蛋白Precursor | 突变范围 Mutation range | 成熟时间 Maturation time/min | 米氏常数 Km/(μmol·L-1) | 催化常数 kcat/(s-1) | 催化常数/米氏常数 kcat/Km/(s-1·mmol-1·L) |
|---|---|---|---|---|---|
| WT | 无 No | 230~240 | 75.69±12.00 | 47.02±7.00 | 621 |
| I-48V | 前导肽 Pro-peptide | 200~210 | 57.68±11.00 | 46.53±6.00 | 807 |
| G-44D | 前导肽 Pro-peptide | 210~220 | 80.45±13.00 | 10.55±3.00 | 131 |
| Y-1D’ | 前导肽 Pro-peptide | 80~90 | 66.68±10.00 | 45.19±5.00 | 678 |
表2 成熟酶催化性能
Table 2 Catalytic properties of active enzymes
| 前体蛋白Precursor | 突变范围 Mutation range | 成熟时间 Maturation time/min | 米氏常数 Km/(μmol·L-1) | 催化常数 kcat/(s-1) | 催化常数/米氏常数 kcat/Km/(s-1·mmol-1·L) |
|---|---|---|---|---|---|
| WT | 无 No | 230~240 | 75.69±12.00 | 47.02±7.00 | 621 |
| I-48V | 前导肽 Pro-peptide | 200~210 | 57.68±11.00 | 46.53±6.00 | 807 |
| G-44D | 前导肽 Pro-peptide | 210~220 | 80.45±13.00 | 10.55±3.00 | 131 |
| Y-1D’ | 前导肽 Pro-peptide | 80~90 | 66.68±10.00 | 45.19±5.00 | 678 |

图6 前体蛋白影响成熟酶活性发挥A:前体蛋白对成熟酶的抑制作用;B:前体蛋白对成熟酶的相对抑制作用
Fig. 6 Precursor proteins affect the activity of mature enzymesA: Precursors inhibited the action of active enzymes; B: Relative inhibition of precursors on enzymes
| 1 | CONTESINI F J, MELO R R, SATO H H. An overview of Bacillus proteases: from production to application [J]. Crit. Rev. Biotechnol., 2018, 38(3): 321-334. |
| 2 | BALLINGER M D, TOM J, WELLS J A. Designing subtilisin BPN’ to cleave substrates containing dibasic residues [J]. Biochemistry, 1995, 34(41): 13312-13319. |
| 3 | IKEMURA H, INOUYE M. In vitro processing of pro-subtilisin in Escherichia coli [J]. J. Biol. Chem., 1988, 263(26): 12959-12963. |
| 4 | CHO J S, OH H J, JANG Y E, et al.. Synthetic pro-peptide design to enhance the secretion of heterologous proteins by Saccharomyces cerevisiae [J/OL]. Microbiol. Open, 2022, 11(3): e1300 [2023-02-12]. . |
| 5 | PENG Z, ZHANG J, SONG Y, et al.. Engineered pro-peptide enhances the catalytic activity of keratinase to improve the conversion ability of feather waste [J]. Biotechnol. Bioeng., 2021, 118(7): 2559-2571. |
| 6 | 罗文. 前导肽对米黑根毛霉脂肪酶的别构调控及其影响机理探究[D]. 广州: 华南农业大学, 2018. |
| LUO W. The allosteric regulation mechanism of Rhizomucor miehei lipase via modification of the propeptide [D]. Guangzhou: South China Agricultural University, 2018. | |
| 7 | 刘柏宏. Bacillus licheniformis角蛋白酶的高效表达、热稳定性及底物特异性改造[D]. 无锡: 江南大学, 2015. |
| LIU B H. Over expression of Bacillus licheniformis keratinase, its molecular modification for enhanced thermostability and substrate specificity [D]. Wuxi: Jiangnan University, 2015. | |
| 8 | INOUYE M, FU X, SHINDE U. Substrate-induced activation of a trapped IMC-mediated protein folding intermediate [J]. Nat. Struct. Mol. Biol., 2001, 8(4): 321-325. |
| 9 | 刘海燕, 张荣珍, 李利宏, 等. 前导肽对Aspergillus pseudoglaucus酸性蛋白酶App表达及功能的影响[J]. 食品与生物技术学, 2020, 39(3): 32-40. |
| LIU H Y, ZHANG R Z, LI L H, et al.. Effects of propeptide on the expression and enzyme function of Aspergillus pseudoglaucus aspartic protease App [J]. J. Food Sci. Biotechnol., 2020, 39(3): 32-40. | |
| 10 | NAGAYAMA M, MAEDA H, KURODA K, et al.. Mutated intramolecular chaperones generate high-activity isomers of mature enzymes [J]. Biochemistry, 2012, 51(17): 3547-3553. |
| 11 | 李丹, 黄非, 夏梦芸, 等. 一新中温碱性蛋白酶基因的克隆及原核表达[J]. 微生物学报, 2013, 53(11): 1240-1250. |
| LI D, HUANG F, XIA M Y, et al.. Molecular cloning and expression of a novel mesophilic alkaline protease from Bacillus sp. L010 in Escherichia coli [J]. Acta Microbiol. Sin., 2013, 53(11): 1240-1250. | |
| 12 | KOSCHORRECK K, SCHMID R D, URLACHER V B. Improving the functional expression of a Bacillus licheniformis laccase by random and site-directed mutagenesis [J/OL]. BMC Biotechnol., 2009, 9:12 [2023-02-12]. . |
| 13 | TAKAGI H, ARAFUKA S, INOUYE M, et al.. The effect of amino acid deletion in subtilisin E, based on structural comparison with a microbial alkaline elastase, on its substrate specificity and catalysis [J]. J. Biochem., 1992, 111(5): 584-588. |
| 14 | LI Y, HU Z, JORDAN F, et al.. Functional analysis of the propeptide of subtilisin E as an intramolecular chaperone for protein folding [J]. J. Biolog. Chem., 1995, 270(42): 25127-25132. |
| 15 | JUNIOR N, CARDOSO M H, CANDIDO E S, et al.. An acidic model pro-peptide affects the secondary structure, membrane interactions and antimicrobial activity of a crotalicidin fragment [J/OL]. Sci. Rep., 2018, 8(1): 11127 [2023-02-12]. . |
| 16 | BALTULIONIS G, BLIGHT M, ROBIN A, et al.. The role of propeptide-mediated autoinhibition and intermolecular chaperone in the maturation of cognate catalytic domain in leucine aminopeptidase [J]. J. Struct. Biol., 2021, 213(3): 107741-107753. |
| 17 | LILIE H, SCHWARZ E, RUDOLPH R. Advances in refolding of proteins produced in E. coli [J]. Curr. Opin. Biotech., 1998, 9(5): 497-501. |
| 18 | KAUR J, SINGH A, PANDA A K, et al.. Protocol for in-vitro purification and refolding of hexachlorocyclohexane degrading enzyme haloalkane dehalogenase LinB from inclusion bodies [J/OL]. Enzyme Microb. Technol., 2021, 146:109760 [2023-02-12]. . |
| 19 | YABUTA Y, TAKAGI H, INOUYE M, et al.. Folding pathway mediated by an intramolecular chaperone: propeptide release modulates activation precision of pro-subtilisin [J]. J. Biol. Chem., 2001, 276(48): 44427-44434. |
| 20 | TAKAGI H, OHTSU I, NAKAMORI S. Construction of novel subtilisin E with high specificity, activity and productivity through multiple amino acid substitutions [J]. Protein Eng., 1997, 10(9): 985-989. |
| 21 | ISHIDA K, SHIMIZU M, WAKASUGI A, et al.. Development of a novel peptide inhibitor of subtilisin BPN’ [J]. FEBS Open Bio., 2022, 12(11): 2057-2064. |
| 22 | BRYAN N B. Protein engineering of subtilisin [J]. Biochim. Biophys. Acta, 2000, 1543(2): 203-222. |
| 23 | FU X, INOUYE M, SHINDE U. Folding pathway mediated by an intramolecular chaperone: the inhibitory and chaperone functions of the subtilisin propeptide are not obligatorily linked [J]. J. Biol. Chem., 2000, 275(22): 16871-16878. |
| 24 | JAIN S C, SHINDE U, LI Y, et al.. The crystal structure of an autoprocessed Ser221Cys-subtilisin E-propeptide complex at 2.0A˚ resolution [J]. J. Mol. Biol., 1998, 284(1): 137-144. |
| 25 | SU C, GONG J S, SUN Y X, et al.. Combining pro-peptide engineering and multisite saturation mutagenesis to improve the catalytic potential of keratinase [J]. ACS Synth. Biol., 2019, 8(2):425-433. |
| 26 | OUYANG X, LIU Y, QU R, et al.. Optimizing protein-glutaminase expression in Bacillus subtilis [J]. Curr. Microbiol., 2021, 78(5):1752-1762. |
| 27 | 彭政. 芽孢杆菌降解羽毛角蛋白关键步骤解析及角蛋白酶的高效表达[D]. 无锡: 江南大学, 2020. |
| PENG Z. Analysis of key steps in degradation of feather keratin by Bacillus and efficient expression of keratinase [D]. Wuxi: Jiangnan University, 2020. | |
| 28 | 沈卫锋, 牛宝龙, 翁宏飚, 等. 枯草芽孢杆菌作为外源基因表达系统的研究进展[J]. 浙江农业学报, 2005, 17(4):234-238. |
| SHEN W F, NIU B L, WENG H B, et al.. The studies on Bacillus subtilis as an expression system of foreign genes [J]. Acta Agric. Zhejiangensis, 2005, 17(4):234-238. |
| [1] | 路泽群, 刘宁, 张红莲, 王苑, 黄火清. 毕赤酵母外源蛋白分泌及折叠途径的改良进展[J]. 中国农业科技导报, 2024, 26(1): 18-27. |
| [2] | 蔡海情, 王微, 曾茂芹, 毕文文, 陈莉, 张黔东, 袁阳, 文明. 疱疹病毒引起内质网应激/未折叠蛋白反应研究进展[J]. 中国农业科技导报, 2023, 25(9): 131-139. |
| [3] | 胡永涛, 汪代斌, 陈益银, 杨超, 郑林林, 史宏志, 王建安. 不同成熟度鲜烟素质对烤后烟叶品质贡献度的研究[J]. 中国农业科技导报, 2023, 25(8): 157-164. |
| [4] | 吴若丁, 何斌, 张亦博, 龚健林, 赵昱权. 双稳态折叠结构日光温室前屋面保温设施的设计与试验[J]. 中国农业科技导报, 2023, 25(5): 112-122. |
| [5] | 刘博远1,赵松超1,李一凡2,贺凡3,阳苇丽4,赵铭钦1*. 不同成熟度雪茄烟晾制过程碳水化合物及相关酶活性变化规律研究[J]. 中国农业科技导报, 2021, 23(4): 192-201. |
| [6] | 吕鹏辉1§,张震东1§,王晔1,周吉红2,宋振伟3,段留生1,李润枝1*. 不同颜色红三叶种子活力的初步研究[J]. 中国农业科技导报, 2021, 23(3): 58-65. |
| [7] | 范宁波1,周俊学2,江凯2,王宏2,史龙飞2,高玉龙3*,陈颐3*. 不同成熟期烤烟主脉中膜脂过氧化及其与衰老相关基因的关系探究[J]. 中国农业科技导报, 2021, 23(3): 66-72. |
| [8] | 赵松超1,田培1,刘博远1,李一凡2,赵铭钦1*. 采收成熟度对雪茄烟叶晾制过程酶促棕色化反应及品质的影响[J]. 中国农业科技导报, 2020, 22(5): 51-59. |
| [9] | 欧爱群1,2,郭娜娜2,刘富海3,刘然3,彭文君2,李江红1*. 蜂蜜成熟过程中主要成分和抑菌特性变化研究[J]. 中国农业科技导报, 2020, 22(2): 101-106. |
| [10] | 马乐,蔡德宝,陈吉宝*. 绿豆RIL群体开花期及成熟期性状的遗传分析[J]. 中国农业科技导报, 2019, 21(12): 33-40. |
| [11] | 袁园园1,常芳2,曹晓慧1,张翼博1,张荣亭1,李斯深3*. 小麦钾效率鉴定和钾高效利用种质的筛选[J]. 中国农业科技导报, 2018, 20(6): 1-10. |
| [12] | 赵喆1,赵东杰1,张蕊2,毛亚博1,牛路路1,周国旺2,张志高2,李燕1,赵铭钦1*. 光照强度对成熟期烤烟衰老生理特性的影响[J]. 中国农业科技导报, 2017, 19(3): 90-97. |
| [13] | 苏丽1,2,逄少军1*,高素芹1. 杂交海带新品系B013孢子囊形成规律初探[J]. 中国农业科技导报, 2017, 19(2): 119-123. |
| [14] | 王栋§,王晨§,马乐,李含笑,陈吉宝*. 甜高粱种子为外植体离体再生体系构建[J]. 中国农业科技导报, 2017, 19(12): 119-125. |
| [15] | 刘磊1,王振忠2*,卢兵友2. 技术成熟度应用现状及加快其在我国农业科技创新应用的政策建议[J]. 中国农业科技导报, 2017, 19(10): 1-6. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号 