




















中国农业科技导报 ›› 2024, Vol. 26 ›› Issue (7): 223-233.DOI: 10.13304/j.nykjdb.2022.1049
• 方法与技术创新 • 上一篇
时晓宇1,2( ), 焦连庆1(
), 焦连庆1( ), 于敏1, 田义新2(
), 于敏1, 田义新2( ), 焦安妮1, 栾依琳2
), 焦安妮1, 栾依琳2
收稿日期:2022-12-01
接受日期:2023-02-01
出版日期:2024-07-15
发布日期:2024-07-12
通讯作者:
焦连庆,田义新
作者简介:时晓宇 E-mail:895592394@qq.com
基金资助:
Xiaoyu SHI1,2( ), Lianqing JIAO1(
), Lianqing JIAO1( ), Min YU1, Yixin TIAN2(
), Min YU1, Yixin TIAN2( ), Anni JIAO1, Yilin LUAN2
), Anni JIAO1, Yilin LUAN2
Received:2022-12-01
Accepted:2023-02-01
Online:2024-07-15
Published:2024-07-12
Contact:
Lianqing JIAO,Yixin TIAN
摘要:
为优化黄芪瞬时高温灭菌(high temperature short time,HTST)工艺参数并多维度考察其对黄芪质量的影响,基于指标相关性的权重系数(criteria importance though intercrieria correlation,CRITIC)法采用正交设计优化黄芪灭菌工艺参数,以灭菌温度、灭菌时间、药材粉碎粒度为考察因素,以灭菌率、5种化合物含量及1,1-二苯基-2-苦基肼自由基(1,1-diphenyl-2-picrylhydrazyl,DPPH·)清除率为考察指标,通过直观及方差分析评价灭菌对3个考察指标的影响;并运用液质联用技术(liquid chromatography-mass spectrometry,LC-MS)确认指纹图谱共有峰的结构,以偏最小二乘回归分析法分析共有峰与抗氧化活性的谱效关系。结果显示, 灭菌温度是具有显著影响的因素(P<0.05),最佳灭菌工艺参数为灭菌温度(170±2)℃,灭菌时间5 s,粉碎粒度50目;按优化工艺灭菌后的3批样品微生物水平均符合药典规定,5种化合物含量及DPPH·清除率与灭菌前比较无明显变化;灭菌前后指纹图谱相似度均大于0.900;谱效学分析相关性结果与化合物单体抗氧化的试验结果及其结构特点基本一致。综上所述,瞬时高温灭菌对黄芪中微生物具有明显杀灭作用且对其质量无明显影响,表明该方法适用于黄芪药材灭菌。
中图分类号:
时晓宇, 焦连庆, 于敏, 田义新, 焦安妮, 栾依琳. 多维度评价及优化黄芪瞬时高温灭菌工艺[J]. 中国农业科技导报, 2024, 26(7): 223-233.
Xiaoyu SHI, Lianqing JIAO, Min YU, Yixin TIAN, Anni JIAO, Yilin LUAN. Multidimensional Evaluation and Optimization of High Temperature Short Time Process of Astragalus membranaceus[J]. Journal of Agricultural Science and Technology, 2024, 26(7): 223-233.
水平 Level | 灭菌温度 Sterilization temperature/℃ | 灭菌时间 Sterilization time/s | 粉碎粒度/目 Fineness of pulverization/mesh |
|---|---|---|---|
| 1 | 170±2 | 10 | 80 |
| 2 | 160±2 | 7 | 50 |
| 3 | 150±2 | 5 | 24 |
表1 正交试验因素水平
Table 1 Level table of orthogonal experimental factors
水平 Level | 灭菌温度 Sterilization temperature/℃ | 灭菌时间 Sterilization time/s | 粉碎粒度/目 Fineness of pulverization/mesh |
|---|---|---|---|
| 1 | 170±2 | 10 | 80 |
| 2 | 160±2 | 7 | 50 |
| 3 | 150±2 | 5 | 24 |
| 时间Time/min | 乙腈 Acetonitrile | 0.2%甲酸水 0.2% formic acid water |
|---|---|---|
| 0—40 | 15%~60% | 85%~40% |
| 40—55 | 60%~75% | 40%~25% |
| 55—56 | 75%~15% | 25%~85% |
| 56—60 | 15% | 85% |
表2 洗脱条件
Table 2 Elution condition
| 时间Time/min | 乙腈 Acetonitrile | 0.2%甲酸水 0.2% formic acid water |
|---|---|---|
| 0—40 | 15%~60% | 85%~40% |
| 40—55 | 60%~75% | 40%~25% |
| 55—56 | 75%~15% | 25%~85% |
| 56—60 | 15% | 85% |
编号 Number | 需氧菌 Aerobic bacteria | 霉菌和酵母菌 Mold and yeast | 大肠埃希菌 E. Coli | 耐胆盐革兰阴性菌 Gallbladder-tolerant gram-negative bacteria | 沙门菌 Salmonella |
|---|---|---|---|---|---|
| 1 | 33×102 | 50 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 2 | 47×102 | 200 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 3 | 34×102 | 200 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 4 | 135×102 | 600 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 5 | 136×102 | 600 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 6 | 99×102 | 300 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 7 | 190×102 | 950 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 8 | 170×102 | 1 100 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 9 | 200×102 | 1 000 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 10 | 105 | 104 | 不可计 Not counted | 不可计 Not counted | 不可计 Not counted |
表3 正交试验黄芪灭菌前后微生物水平考察结果 (CFU·g-1)
Table 3 Results of orthogonal test on microorganism level of Astragalusme membranaceus before and after sterilization
编号 Number | 需氧菌 Aerobic bacteria | 霉菌和酵母菌 Mold and yeast | 大肠埃希菌 E. Coli | 耐胆盐革兰阴性菌 Gallbladder-tolerant gram-negative bacteria | 沙门菌 Salmonella |
|---|---|---|---|---|---|
| 1 | 33×102 | 50 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 2 | 47×102 | 200 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 3 | 34×102 | 200 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 4 | 135×102 | 600 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 5 | 136×102 | 600 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 6 | 99×102 | 300 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 7 | 190×102 | 950 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 8 | 170×102 | 1 100 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 9 | 200×102 | 1 000 | 未检出 Not detected | 未检出 Not detected | 未检出 Not detected |
| 10 | 105 | 104 | 不可计 Not counted | 不可计 Not counted | 不可计 Not counted |
| 样品编号Sample number | 毛蕊异黄酮苷 Calycosin-7-glucoside | 毛蕊异黄酮 Calycosin | 山奈酚 Kaempferol | 芒柄花苷 Ononin | 刺芒柄花素Formononetin | 总含量 Total content |
|---|---|---|---|---|---|---|
| 1 | 0.040 | 0.003 | 0.032 | 0.021 | 0.011 | 0.107 |
| 2 | 0.057 | 0.004 | 0.041 | 0.035 | 0.012 | 0.149 |
| 3 | 0.058 | 0.004 | 0.044 | 0.036 | 0.013 | 0.155 |
| 4 | 0.037 | 0.003 | 0.029 | 0.034 | 0.010 | 0.113 |
| 5 | 0.036 | 0.004 | 0.034 | 0.030 | 0.012 | 0.116 |
| 6 | 0.058 | 0.004 | 0.036 | 0.034 | 0.013 | 0.145 |
| 7 | 0.038 | 0.003 | 0.034 | 0.033 | 0.010 | 0.118 |
| 8 | 0.037 | 0.003 | 0.034 | 0.024 | 0.011 | 0.109 |
| 9 | 0.044 | 0.004 | 0.037 | 0.020 | 0.013 | 0.118 |
表4 正交试验黄芪5种化合物含量 (%)
Table 4 Content of 5 kinds of compounds in Astragalus membranaceus by orthogonal test
| 样品编号Sample number | 毛蕊异黄酮苷 Calycosin-7-glucoside | 毛蕊异黄酮 Calycosin | 山奈酚 Kaempferol | 芒柄花苷 Ononin | 刺芒柄花素Formononetin | 总含量 Total content |
|---|---|---|---|---|---|---|
| 1 | 0.040 | 0.003 | 0.032 | 0.021 | 0.011 | 0.107 |
| 2 | 0.057 | 0.004 | 0.041 | 0.035 | 0.012 | 0.149 |
| 3 | 0.058 | 0.004 | 0.044 | 0.036 | 0.013 | 0.155 |
| 4 | 0.037 | 0.003 | 0.029 | 0.034 | 0.010 | 0.113 |
| 5 | 0.036 | 0.004 | 0.034 | 0.030 | 0.012 | 0.116 |
| 6 | 0.058 | 0.004 | 0.036 | 0.034 | 0.013 | 0.145 |
| 7 | 0.038 | 0.003 | 0.034 | 0.033 | 0.010 | 0.118 |
| 8 | 0.037 | 0.003 | 0.034 | 0.024 | 0.011 | 0.109 |
| 9 | 0.044 | 0.004 | 0.037 | 0.020 | 0.013 | 0.118 |
样品编号 Sample number | 灭菌温度 Sterilization temperature/℃ | 灭菌时间 Sterilization time/s | 粉碎粒度/目 Fineness of pulverization/mesh | 空白 Blank | 灭菌率 Sterilization rate/% | 5种化合物 总含量 Total content of 5 compounds/% | DPPH• 清除率 DPPH•clearance rate/% | 综合评分Synthesize score | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 170±2 | 10 | 80 | 96.95 | 0.107 | 52.29 | 0.866 | ||
| 2 | 170±2 | 7 | 50 | 95.50 | 0.149 | 62.89 | 0.964 | ||
| 3 | 170±2 | 5 | 24 | 96.75 | 0.155 | 67.79 | 0.999 | ||
| 4 | 160±2 | 10 | 24 | 87.20 | 0.113 | 53.96 | 0.832 | ||
| 5 | 160±2 | 7 | 80 | 87.15 | 0.116 | 57.95 | 0.852 | ||
| 6 | 160±2 | 5 | 50 | 90.80 | 0.145 | 60.20 | 0.923 | ||
| 7 | 150±2 | 10 | 50 | 81.85 | 0.118 | 58.88 | 0.831 | ||
| 8 | 150±2 | 7 | 24 | 83.50 | 0.109 | 55.53 | 0.813 | ||
| 9 | 150±2 | 5 | 80 | 80.93 | 0.118 | 59.01 | 0.827 | ||
灭菌率 Sterilization rate/% | K1 | 96.333 | 88.600 | 88.277 | 90.350 | — | — | — | — |
| K2 | 88.383 | 88.717 | 89.383 | 87.877 | — | — | — | — | |
| K3 | 82.093 | 89.493 | 89.150 | 88.583 | — | — | — | — | |
| 极差R | 14.240 | 0.893 | 1.106 | 2.473 | — | — | — | — | |
| 5种化合物总含量 Total content of 5 compounds/% | K1 | 0.137 | 0.113 | 0.114 | 0.120 | — | — | — | — |
| K2 | 0.125 | 0.125 | 0.137 | 0.127 | — | — | — | — | |
| K3 | 0.115 | 0.139 | 0.126 | 0.130 | — | — | — | — | |
| 极差R | 0.022 | 0.026 | 0.023 | 0.010 | — | — | — | — | |
DPPH•清除率 DPPH•clearance rate/% | K1 | 60.990 | 55.043 | 56.417 | 56.007 | — | — | — | — |
| K2 | 57.370 | 58.790 | 60.657 | 58.620 | — | — | — | — | |
| K3 | 57.807 | 62.333 | 59.093 | 61.540 | — | — | — | — | |
| 极差R | 3.620 | 7.290 | 4.240 | 5.533 | — | — | — | — | |
综合评分 Synthesize score | K1 | 0.943 | 0.876 | 0.848 | 0.867 | — | — | — | — |
| K2 | 0.869 | 0.876 | 0.939 | 0.874 | — | — | — | — | |
| K3 | 0.857 | 0.916 | 0.881 | 0.927 | — | — | — | — | |
| 极差R | 0.086 | 0.040 | 0.091 | 0.060 | — | — | — | — | |
表5 瞬时高温灭菌工艺 L9(34)正交试验设计及结果
Table 5 Orthogonal test design and results of instantaneous high temperature sterilization process L9(34)
样品编号 Sample number | 灭菌温度 Sterilization temperature/℃ | 灭菌时间 Sterilization time/s | 粉碎粒度/目 Fineness of pulverization/mesh | 空白 Blank | 灭菌率 Sterilization rate/% | 5种化合物 总含量 Total content of 5 compounds/% | DPPH• 清除率 DPPH•clearance rate/% | 综合评分Synthesize score | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 170±2 | 10 | 80 | 96.95 | 0.107 | 52.29 | 0.866 | ||
| 2 | 170±2 | 7 | 50 | 95.50 | 0.149 | 62.89 | 0.964 | ||
| 3 | 170±2 | 5 | 24 | 96.75 | 0.155 | 67.79 | 0.999 | ||
| 4 | 160±2 | 10 | 24 | 87.20 | 0.113 | 53.96 | 0.832 | ||
| 5 | 160±2 | 7 | 80 | 87.15 | 0.116 | 57.95 | 0.852 | ||
| 6 | 160±2 | 5 | 50 | 90.80 | 0.145 | 60.20 | 0.923 | ||
| 7 | 150±2 | 10 | 50 | 81.85 | 0.118 | 58.88 | 0.831 | ||
| 8 | 150±2 | 7 | 24 | 83.50 | 0.109 | 55.53 | 0.813 | ||
| 9 | 150±2 | 5 | 80 | 80.93 | 0.118 | 59.01 | 0.827 | ||
灭菌率 Sterilization rate/% | K1 | 96.333 | 88.600 | 88.277 | 90.350 | — | — | — | — |
| K2 | 88.383 | 88.717 | 89.383 | 87.877 | — | — | — | — | |
| K3 | 82.093 | 89.493 | 89.150 | 88.583 | — | — | — | — | |
| 极差R | 14.240 | 0.893 | 1.106 | 2.473 | — | — | — | — | |
| 5种化合物总含量 Total content of 5 compounds/% | K1 | 0.137 | 0.113 | 0.114 | 0.120 | — | — | — | — |
| K2 | 0.125 | 0.125 | 0.137 | 0.127 | — | — | — | — | |
| K3 | 0.115 | 0.139 | 0.126 | 0.130 | — | — | — | — | |
| 极差R | 0.022 | 0.026 | 0.023 | 0.010 | — | — | — | — | |
DPPH•清除率 DPPH•clearance rate/% | K1 | 60.990 | 55.043 | 56.417 | 56.007 | — | — | — | — |
| K2 | 57.370 | 58.790 | 60.657 | 58.620 | — | — | — | — | |
| K3 | 57.807 | 62.333 | 59.093 | 61.540 | — | — | — | — | |
| 极差R | 3.620 | 7.290 | 4.240 | 5.533 | — | — | — | — | |
综合评分 Synthesize score | K1 | 0.943 | 0.876 | 0.848 | 0.867 | — | — | — | — |
| K2 | 0.869 | 0.876 | 0.939 | 0.874 | — | — | — | — | |
| K3 | 0.857 | 0.916 | 0.881 | 0.927 | — | — | — | — | |
| 极差R | 0.086 | 0.040 | 0.091 | 0.060 | — | — | — | — | |
因素 Factor | 平方和 Sum of square | 自由度 Degree of freedom | 均方 Mean square | F值 F value | 显著性 Significance | |
|---|---|---|---|---|---|---|
灭菌率 Sterilization rate | 灭菌温度 Sterilization temperature | 308.512 | 2 | 154.256 | 29.917 | 0.032 |
灭菌时间 Sterilization time | 1.289 | 2 | 0.645 | 0.125 | 0.889 | |
粉碎粒度 Fineness of pulverization | 1.787 | 2 | 0.893 | 0.173 | 0.852 | |
| 误差Error | 10.312 | 2 | 5.156 | — | — | |
5种化合物总含量 Total content of 5 compounds | 灭菌温度 Sterilization temperature | 0.159 | 2 | 0.079 | 3.366 | 0.157 |
灭菌时间 Sterilization time | 0.105 | 2 | 0.052 | 2.226 | 0.113 | |
粉碎粒度 Fineness of pulverization | 0.251 | 2 | 0.125 | 5.324 | 0.140 | |
| 误差Error | 0.047 | 2 | 0.024 | — | — | |
DPPH•清除率 DPPH• clearance rate | 灭菌温度 Sterilization temperature | 1.269 | 2 | 0.063 | 7.372 | 0.662 |
灭菌时间 Sterilization time | 0.508 | 2 | 0.254 | 2.950 | 0.366 | |
粉碎粒度 Fineness of pulverization | 0.169 | 2 | 0.084 | 0.981 | 0.625 | |
| 误差Error | 0.172 | 2 | 0.086 | — | — | |
综合评分 Synthesize score | 灭菌温度 Sterilization temperature | 0.009 | 2 | 0.004 | 20.751 | 0.050 |
灭菌时间 Sterilization time | 0.003 | 2 | 0.001 | 6.443 | 0.124 | |
粉碎粒度 Fineness of pulverization | 0.004 | 2 | 0.002 | 8.713 | 0.186 | |
| 误差Error | 0.000 | 2 | 0.000 | — | — | |
表6 瞬时高温灭菌工艺正交试验方差分析表
Table 6 Table of variance analysis of orthogonal test of high temperature short time process
因素 Factor | 平方和 Sum of square | 自由度 Degree of freedom | 均方 Mean square | F值 F value | 显著性 Significance | |
|---|---|---|---|---|---|---|
灭菌率 Sterilization rate | 灭菌温度 Sterilization temperature | 308.512 | 2 | 154.256 | 29.917 | 0.032 |
灭菌时间 Sterilization time | 1.289 | 2 | 0.645 | 0.125 | 0.889 | |
粉碎粒度 Fineness of pulverization | 1.787 | 2 | 0.893 | 0.173 | 0.852 | |
| 误差Error | 10.312 | 2 | 5.156 | — | — | |
5种化合物总含量 Total content of 5 compounds | 灭菌温度 Sterilization temperature | 0.159 | 2 | 0.079 | 3.366 | 0.157 |
灭菌时间 Sterilization time | 0.105 | 2 | 0.052 | 2.226 | 0.113 | |
粉碎粒度 Fineness of pulverization | 0.251 | 2 | 0.125 | 5.324 | 0.140 | |
| 误差Error | 0.047 | 2 | 0.024 | — | — | |
DPPH•清除率 DPPH• clearance rate | 灭菌温度 Sterilization temperature | 1.269 | 2 | 0.063 | 7.372 | 0.662 |
灭菌时间 Sterilization time | 0.508 | 2 | 0.254 | 2.950 | 0.366 | |
粉碎粒度 Fineness of pulverization | 0.169 | 2 | 0.084 | 0.981 | 0.625 | |
| 误差Error | 0.172 | 2 | 0.086 | — | — | |
综合评分 Synthesize score | 灭菌温度 Sterilization temperature | 0.009 | 2 | 0.004 | 20.751 | 0.050 |
灭菌时间 Sterilization time | 0.003 | 2 | 0.001 | 6.443 | 0.124 | |
粉碎粒度 Fineness of pulverization | 0.004 | 2 | 0.002 | 8.713 | 0.186 | |
| 误差Error | 0.000 | 2 | 0.000 | — | — | |
序号 Number | 需氧菌 Aerobic bacteria | 霉菌和酵母菌 Mold and yeast | 大肠埃希菌 E. Coli/ | 耐胆盐革兰阴性菌 Gallbladder-tolerant gram-negative bacteria | 沙门菌 Salmonella |
|---|---|---|---|---|---|
| S1 | 1.2×103 | 50 | 未检出 Not detected | <10 | 未检出 Not detected |
| S2 | 1.1×103 | 40 | 未检出 Not detected | <10 | 未检出 Not detected |
| S3 | 1.4×103 | 43 | 未检出 Not detected | <10 | 未检出 Not detected |
| 未灭菌Unsterilized | 1.0×105 | 1.0×104 | 未检出 Not detected | <10 | 未检出 Not detected |
表7 灭菌工艺处理前后微生物水平考察 (CFU·g-1)
Table 7 Microbial standard before and after sterilization of the process
序号 Number | 需氧菌 Aerobic bacteria | 霉菌和酵母菌 Mold and yeast | 大肠埃希菌 E. Coli/ | 耐胆盐革兰阴性菌 Gallbladder-tolerant gram-negative bacteria | 沙门菌 Salmonella |
|---|---|---|---|---|---|
| S1 | 1.2×103 | 50 | 未检出 Not detected | <10 | 未检出 Not detected |
| S2 | 1.1×103 | 40 | 未检出 Not detected | <10 | 未检出 Not detected |
| S3 | 1.4×103 | 43 | 未检出 Not detected | <10 | 未检出 Not detected |
| 未灭菌Unsterilized | 1.0×105 | 1.0×104 | 未检出 Not detected | <10 | 未检出 Not detected |
| 序号Number | 5种化合物总含量Total content of 5 compounds | DPPH·清除率DPPH· clearance | ||||
|---|---|---|---|---|---|---|
| 灭菌前 Before sterilization | 灭菌后 After sterilization | 变幅 Range | 灭菌前 Before sterilization | 灭菌后 After sterilization | 变幅 Range | |
| S1 | 0.153 | 0.155 | 0.002 | 62.00 | 63.07 | 1.07 |
| S2 | 0.141 | 0.145 | 0.004 | 57.47 | 58.87 | 1.40 |
| S3 | 0.148 | 0.151 | 0.003 | 52.95 | 53.96 | 1.01 |
| 平均值Average | 0.147 | 0.150 | 0.003 | 57.47 | 58.63 | 1.16 |
表8 灭菌工艺处理前后5种化合物总含量及DPPH?清除率 (%)
Table 8 Total content of 5 compounds and DPPH· clearance before and after sterilization process
| 序号Number | 5种化合物总含量Total content of 5 compounds | DPPH·清除率DPPH· clearance | ||||
|---|---|---|---|---|---|---|
| 灭菌前 Before sterilization | 灭菌后 After sterilization | 变幅 Range | 灭菌前 Before sterilization | 灭菌后 After sterilization | 变幅 Range | |
| S1 | 0.153 | 0.155 | 0.002 | 62.00 | 63.07 | 1.07 |
| S2 | 0.141 | 0.145 | 0.004 | 57.47 | 58.87 | 1.40 |
| S3 | 0.148 | 0.151 | 0.003 | 52.95 | 53.96 | 1.01 |
| 平均值Average | 0.147 | 0.150 | 0.003 | 57.47 | 58.63 | 1.16 |
| 指标 Index | 灭菌前后 Before and after sterilization | 平均值 Average | 标准差Standard deviation | F值 F value | P值 P value | t值 t value | 自由度 Free degree | |
|---|---|---|---|---|---|---|---|---|
5种化合物总含量 Total content of 5 compounds/% | 灭菌前 Before sterilization | 0.147 | 0.006 | 假定等方差 Assumed isovariance | 0.084 | 0.787 | -0.662 | 4 |
灭菌后 After sterilization | 0.150 | 0.005 | ||||||
DPPH·清除率 DPPH· clearance/% | 灭菌前 Before sterilization | 57.47 | 4.53 | 假定等方差 Assumed isovariance | 0.002 | 0.965 | -0.313 | 4 |
灭菌后 After sterilization | 58.63 | 4.56 |
表9 灭菌工艺处理前后5种化合物总含量及DPPH?清除率结果t检验
Table 9 t test of the total content of 5 compounds and the DPPH· clearance rate before and after the sterilization process
| 指标 Index | 灭菌前后 Before and after sterilization | 平均值 Average | 标准差Standard deviation | F值 F value | P值 P value | t值 t value | 自由度 Free degree | |
|---|---|---|---|---|---|---|---|---|
5种化合物总含量 Total content of 5 compounds/% | 灭菌前 Before sterilization | 0.147 | 0.006 | 假定等方差 Assumed isovariance | 0.084 | 0.787 | -0.662 | 4 |
灭菌后 After sterilization | 0.150 | 0.005 | ||||||
DPPH·清除率 DPPH· clearance/% | 灭菌前 Before sterilization | 57.47 | 4.53 | 假定等方差 Assumed isovariance | 0.002 | 0.965 | -0.313 | 4 |
灭菌后 After sterilization | 58.63 | 4.56 |
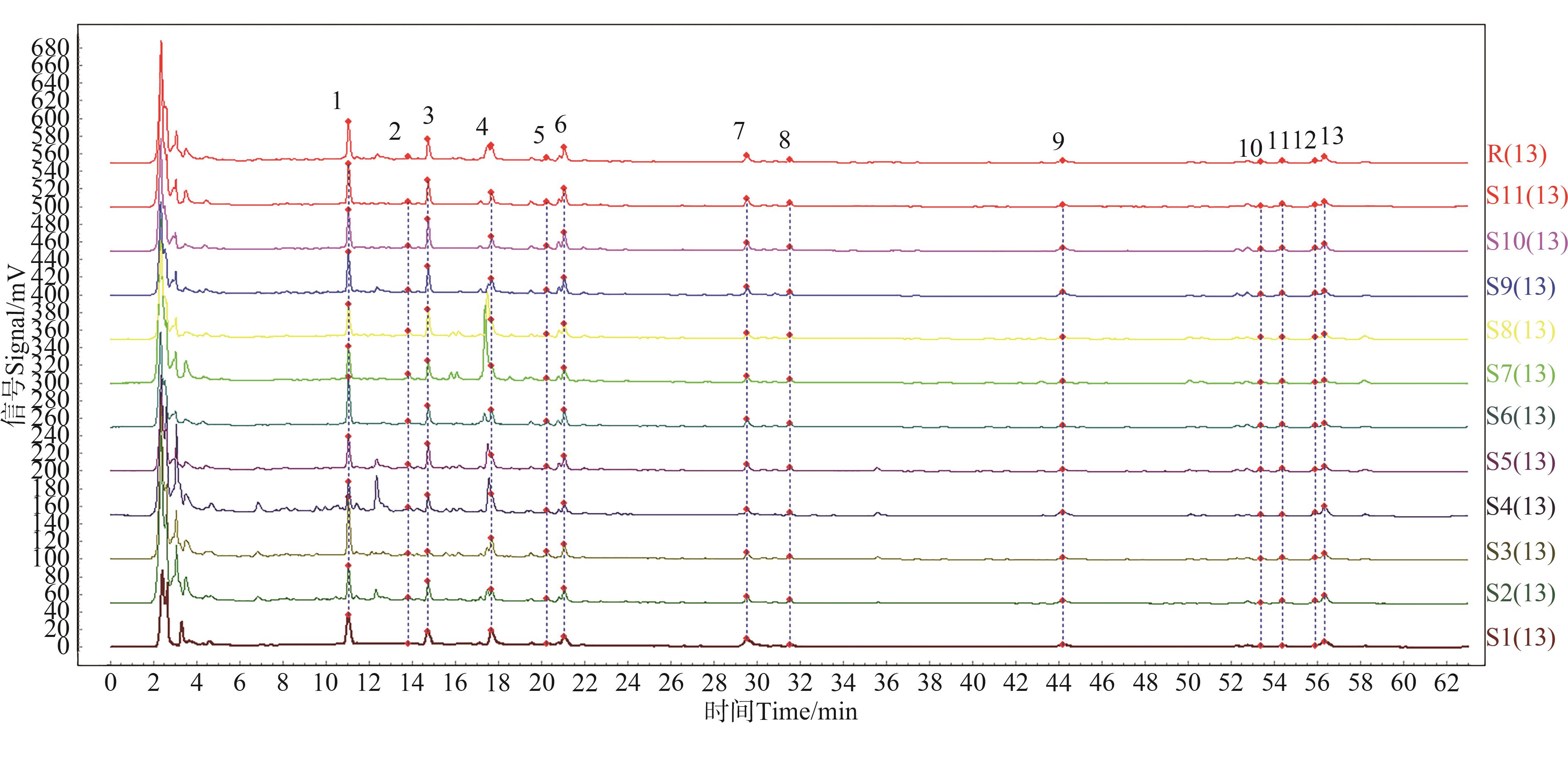
图1 黄芪的HPLC指纹图谱及对照指纹图谱注:1—毛蕊异黄酮苷;4—芒柄花苷;5—毛蕊异黄酮;6—山奈酚;7—刺芒柄花素;R—对照图谱;S1~S10—灭菌后样品;S11—未灭菌样品。
Fig. 1 HPLC fingerprint and control fingerprint of Astragalus membranaceusNote:1—Calycosin-7-O-bate-glucoside;4—Ononin;5—Calycosin;6—Kaempferol;7—Formononetin;R—Control map; S1~S10—Sterilized samples; S11—Unsterilized sample.
峰号 Peak number | 化合物 Compound | 分子式 Molecular formula | 相对分子质量 Relative molecular mass | 二级碎片离子 Secondary fragment ion/m/z |
|---|---|---|---|---|
| 1 | 毛蕊异黄酮苷Calycosin-7-glucoside | C22H22NO10 | 446.40 | 285[M-Glu]+,447[M+H]+ |
| 2 | 毛蕊异黄酮-丙二酸盐异构体 Isomer of calycosin-glu-malonate | C25H24O13 | 532.00 | 285[M-Glu-C3HO3]+,533[M+H]+ |
| 3 | 毛蕊异黄酮-7-O-葡萄糖-6"-O-丙二酸盐 Calycosin-7-O-glu-6"-O-malonate | C25H24O13 | 532.00 | 285[M-Glu-C3HO3]+,489[M-CH3CO]+ |
| 4 | 芒柄花苷Ononin | C22H22O9 | 430.40 | 269[M-Glu]+,431[M+H]+ |
| 5 | 毛蕊异黄酮Calycosin | C16H12O5 | 284.26 | 285[M+H]+ |
| 6 | 山奈酚Kaempferol | C15H10O6 | 286.23 | 285[M-H]-,256[M-H-CO]- |
| 7 | 刺芒柄花素Formononetin | C16H12O4 | 268.26 | 269[M+H]+,270[M+2H]+ |
| 8 | 鼠李柠檬素Rhamnocitrin | C16H12O6 | 300.27 | 301[M+H]+ |
| 9 | 7,2’-二羟基-3’,4’-二甲氧基异黄烷 7,2’-dihydroxy-3’,4’-dimethoxyisoflavan | C17H18O5 | 302.32 | 303[M+H]+,325[M+Na]+ |
| 10 | 熊竹素Kumatakenin | C17H14O6 | 314.29 | 337[M+Na]+ |
| 11 | 汉黄芩素/千层纸素Wogonin/oroxylin A | C16H12O5 | 284.26 | 603[2M+Cl]- |
| 12 | 异鼠李素Isorhamnetin | C16H12O7 | 316.26 | 317[M+H]+ |
| 13 | (6αR,11αR)-10-羟基-3,9-二甲氧基紫檀烷 3-hydroxy-9,10-dimethoxypterocarpan | C17H16O5 | 300.10 | 301[M+H]+ |
表10 黄芪13个共有峰鉴定结果
Table 10 Mass spectrum information of 13 common peaks in Astragalus membranaceus
峰号 Peak number | 化合物 Compound | 分子式 Molecular formula | 相对分子质量 Relative molecular mass | 二级碎片离子 Secondary fragment ion/m/z |
|---|---|---|---|---|
| 1 | 毛蕊异黄酮苷Calycosin-7-glucoside | C22H22NO10 | 446.40 | 285[M-Glu]+,447[M+H]+ |
| 2 | 毛蕊异黄酮-丙二酸盐异构体 Isomer of calycosin-glu-malonate | C25H24O13 | 532.00 | 285[M-Glu-C3HO3]+,533[M+H]+ |
| 3 | 毛蕊异黄酮-7-O-葡萄糖-6"-O-丙二酸盐 Calycosin-7-O-glu-6"-O-malonate | C25H24O13 | 532.00 | 285[M-Glu-C3HO3]+,489[M-CH3CO]+ |
| 4 | 芒柄花苷Ononin | C22H22O9 | 430.40 | 269[M-Glu]+,431[M+H]+ |
| 5 | 毛蕊异黄酮Calycosin | C16H12O5 | 284.26 | 285[M+H]+ |
| 6 | 山奈酚Kaempferol | C15H10O6 | 286.23 | 285[M-H]-,256[M-H-CO]- |
| 7 | 刺芒柄花素Formononetin | C16H12O4 | 268.26 | 269[M+H]+,270[M+2H]+ |
| 8 | 鼠李柠檬素Rhamnocitrin | C16H12O6 | 300.27 | 301[M+H]+ |
| 9 | 7,2’-二羟基-3’,4’-二甲氧基异黄烷 7,2’-dihydroxy-3’,4’-dimethoxyisoflavan | C17H18O5 | 302.32 | 303[M+H]+,325[M+Na]+ |
| 10 | 熊竹素Kumatakenin | C17H14O6 | 314.29 | 337[M+Na]+ |
| 11 | 汉黄芩素/千层纸素Wogonin/oroxylin A | C16H12O5 | 284.26 | 603[2M+Cl]- |
| 12 | 异鼠李素Isorhamnetin | C16H12O7 | 316.26 | 317[M+H]+ |
| 13 | (6αR,11αR)-10-羟基-3,9-二甲氧基紫檀烷 3-hydroxy-9,10-dimethoxypterocarpan | C17H16O5 | 300.10 | 301[M+H]+ |
| 1 | 国家药典委员会. 中华人民共和国药典: 2020年版[M]. 北京: 中国医药科技出版社, 2020: 1088. |
| 2 | 张蔷, 高文远, 满淑丽. 黄芪中有效成分药理活性的研究进展[J]. 中国中药杂志,2012, 37(21): 3203-3207. |
| ZHANG Q, GAO W Y, MAN S L. Chemical composition and pharmacological activities of Astragali Radix [J]. China J. Chin. Materia Med., 2012, 37(21): 3203-3207. | |
| 3 | ZHENG Y J, REN W Y, ZHANG L N, et al.. A review of the pharmacological action of astragalus polysaccharide [J/OL]. Front. Pharmacol., 2020,11:349 [2022-11-01]. . |
| 4 | LIU P, ZHAO H, LUO Y. Anti-aging implications of Astragalus membranaceus (Huangqi): a well-known chinese tonic [J]. Aging Dis., 2017, 8(6): 868-886. |
| 5 | 滕宝霞, 牟建平, 贺晓文. 5种不同灭菌方式对黄芪饮片中毛蕊异黄酮葡萄糖苷含量的影响[J]. 甘肃医药, 2019, 38(6):544-546. |
| 6 | 孔祥山,邵晓慧, 林海岩. 中药制剂灭菌技术的应用初探[J].山东医药工业, 2002, 21(1): 39-40. |
| 7 | 胡彦君, 王雅琪, 伍振峰, 等. 臭氧灭菌技术在中药及其制剂应用中的研究进展[J]. 中国中药杂志, 2015, 40(16): 3137-3141. |
| HU Y J, WANG Y Q, WU Z F, et al.. Preliminary study on suitability of ozone sterilization in traditional Chinese medicine and its preparation [J]. China J. Chin. Materia Med., 2015, 40(16): 3137-3141. | |
| 8 | 张立雯, 林玲, 梁伟洪,等. 干热、湿热、辐照对龙胆和秦艽化学成分和灭菌效果的比较研究[J]. 中药新药与临床药理,2016, 27(5): 692-697. |
| ZHANG L W, LIN L, LIANG W H, et al.. Effects of dry heat, moist heat and cobalt-60 sterilization on chemical composition and microbial killing of radix gentianae and radix gentianae macrophyllae [J]. Trad. Chin. Drug Res. Clinical Pharmacol., 2016, 27(5): 692-697. | |
| 9 | 林彤, 毕福钧, 吕渭升, 等. 中药的辐照灭菌现状与监管[J].中国药学杂志, 2019, 54(17): 1442-1447. |
| LIN T, BI F J, LYU W S, et al.. Status and control of radicidation of tranditional Chinese medicine [J]. Chin. Pharm. J., 2019, 54(17): 1442-1447. | |
| 10 | 尚海宾, 陶海涛, 乔晓芳. 中药高温瞬时灭菌设备的智能化设计与性能确认[J]. 流程工业, 2022(7): 58-61. |
| 11 | 黄定轩. 基于客观信息熵的多因素权重分配方法[J]. 系统工程理论方法应用, 2003, 12(4): 321-324. |
| HUANG D X. Means of weights allocation with multi-factors based on impersonal message entropy [J]. Syst. Eng. Theory Methodol. Appl.,2003,12(4):321-324. | |
| 12 | 中华人民共和国药典委员会. 中华人民共和国药典(四部) [M]. 北京: 中国医药科技出版社, 2020: 160-179. |
| 13 | 康超超, 王学成, 伍振峰, 等. 当归原生粉乙醇灭菌工艺优化及其品质比较研究 [J]. 中草药, 2019, 50(6): 1341-1347. |
| KANG C C, WANG X C, WU Z F, et al.. Optimization of ethanol sterilization technology for Angelicae Sinensis Radix and comparison of its quality [J]. China Tradit. Herb. Drugs, 2019, 50(6): 1341-1347. | |
| 14 | 陈伯丛,王汝上,罗德祥.不同产地黄芪总黄酮HPLC指纹图谱研究[J].北方药学,2016,13(7):8-10. |
| CHEN B C, WANG R S, LUO D X. HPLC fingerprinting of flavonoids in radix astragali [J]. J. North Pharmacy, 2016,13(7):8-10. | |
| 15 | SHARMA O P, BHAT T K. DPPH antioxidant assay revisited [J]. Food Chem., 2009,113(4): 1202-1205. |
| 16 | YANG B, ZHAO M M, SHI J, et al.. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp [J]. Food Chem., 2008,106(2):685-690. |
| 17 | 王存琴,陈颖,汪雷,等. HPLC同时测定大叶冬青叶中10种黄酮成分的含量及抗氧化活性研究[J].天然产物研究与开发,2019,31(4):557-565. |
| WANG C Q, CHEN Y, WANG L,et al.. Determination of 10 polyphenol components by HPLC and antioxidant activity in the leaves of Ilex latifolia [J]. Nat. Prod. Res. Dev., 2019,31 (4):557-565. | |
| 18 | 王强,张湛,杨彬,等.基于AHP-CRITIC混合加权法的正交试验优选神气汤提取工艺[J].中国药业,2021,30(19):51-55. |
| WANG Q, ZHANG Z, YANG B, et al..Optimization of extraction process of Shenqi decoction by the orthogonal test based on AHP-CRITIC mixed weighting method [J]. China Pharm., 2021, 30(19):51-55. | |
| 19 | 冯伟红,李春,信伟梅,等.生物测定法用于中药质量评价的探索研究——以夏枯草抗氧化活性与总酚酸含量相关性的研究为例[J].中国中药杂志,2016,41(14):2660-2668. |
| FENG W H, LI C, XIN W M, et al.. Exploration on feasibility of introducing bioassay method into quality evaluation of Chinese herbal medicines by studying on the correlation between antioxidant activity of Prunella vulgaris and its total phenolic acids content for example [J]. China J. Chin. Materia Med., 2016,41(14):2660-2668. | |
| 20 | 张靖. 黄芪药对及复方的化学成分质谱学鉴定及多成分定量研究[D]. 广州: 广州中医药大学, 2015. |
| ZHANG J. The study on mass spectrometry identification and multi-component quantitative studies of Huangqi herb pair and its compound preparation [D]. Guangzhou: Guangzhou University of Chinese Medicine, 2015. | |
| 21 | 谭雪霞, 朱宏明, 唐铖, 等. HPLC-MS鉴定黄芪水煎液中黄酮类成分[J]. 现代药物与临床, 2021, 36(2): 231-235. |
| TAN X X, ZHU H M, TANG C, et al.. HPLC-MS identification on flavonoids in Astragali radix decoction [J]. Drugs Clinic, 2021, 36(2): 231-235. | |
| 22 | 周颖, 黄锐. 超高温瞬时灭菌工艺优化[J]. 中国乳业, 2020(2): 81-84. |
| 23 | 袁武会. 参术止带糖浆的灭菌工艺研究[J]. 中国药业,2013,22(2): 25-27. |
| 24 | 李振豪, 李顺仓, 王杰, 等. 不同灭菌法对婴儿健脾散成分及微生物的影响[J]. 流程工业, 2020(6): 56-59. |
| 25 | 张婷, 焦连庆, 刘融融, 等. 基于多指标分析优化苦丁茶冬青瞬时高温灭菌工艺[J]. 食品工业科技, 2023, 44(8):205-211. |
| ZHANG T, JIAO L Q, LIU R R, et al.. Optimize the instantaneous high temperature sterilization process of Ilex kudingcha based on multiple index analysis [J]. Sci. Technol. Food Ind, 2023, 44(8): 205-211 . | |
| 26 | 姚静, 杨晓宁, 朱平, 等. HPLC-CAD指纹图谱结合化学计量学评价不同产地黄芪质量[J]. 中成药, 2022, 44(10): 3214-3219. |
| YAO J, YANG X N, ZHU P, et al.. Quality evaluation of Astragali Radix from different producing areas by HPLC⁃ CAD fingerprints combined with chemometrics [J]. Chin. Tradit. Patent Med., 2022, 44(10): 3214-3219. | |
| 27 | 陈星宇, 谭魏, 汪虹, 等. 黄芪黄酮抗氧化活性的构效关系分析[J]. 广州化工, 2021, 49(24): 26-30. |
| CHEN X Y, TAN W, WANG H, et al.. Structure-activity relationship analysis on antioxidant activity of astragalus flavonoidss [J]. Guangzhou Chem. Ind., 2021,49(24):26-30. |
| [1] | 刘融融, 焦连庆, 张婷, 于敏, 田义新. 基于CRITIC法优化大叶冬青瞬时高温灭菌工艺[J]. 中国农业科技导报, 2023, 25(12): 205-215. |
| [2] | 李紫岩1,朱寿东2,刘澜波3,杨敏1,张磊4,张春红1*,李旻辉1,4*. 内蒙古道地药材蒙古黄芪生态适宜性区划研究[J]. 中国农业科技导报, 2021, 23(2): 170-176. |
| [3] | 高天明,闫志坚,高丽. 四种沙漠植物的抗旱研究[J]. , 2008, 10(2): 105-109. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号