




















Journal of Agricultural Science and Technology ›› 2024, Vol. 26 ›› Issue (6): 214-225.DOI: 10.13304/j.nykjdb.2023.0098
• INNOVATIVE METHODS AND TECHNOLOGIES • Previous Articles
Xiaoxiao ZHANG( ), Xiaoqian LI, Cheng ZHU, Chenze LYU(
), Xiaoqian LI, Cheng ZHU, Chenze LYU( )
)
Received:2023-02-16
Accepted:2023-05-05
Online:2024-06-15
Published:2024-06-12
Contact:
Chenze LYU
通讯作者:
吕晨泽
作者简介:张笑笑 E-mail: 812464699@qq.com;
基金资助:CLC Number:
Xiaoxiao ZHANG, Xiaoqian LI, Cheng ZHU, Chenze LYU. Research Status and Development Trend of Rapid Detection of Agglutinin in Concanavalin A[J]. Journal of Agricultural Science and Technology, 2024, 26(6): 214-225.
张笑笑, 李晓倩, 朱诚, 吕晨泽. 刀豆凝集素快速检测技术的研究现状及发展趋势[J]. 中国农业科技导报, 2024, 26(6): 214-225.
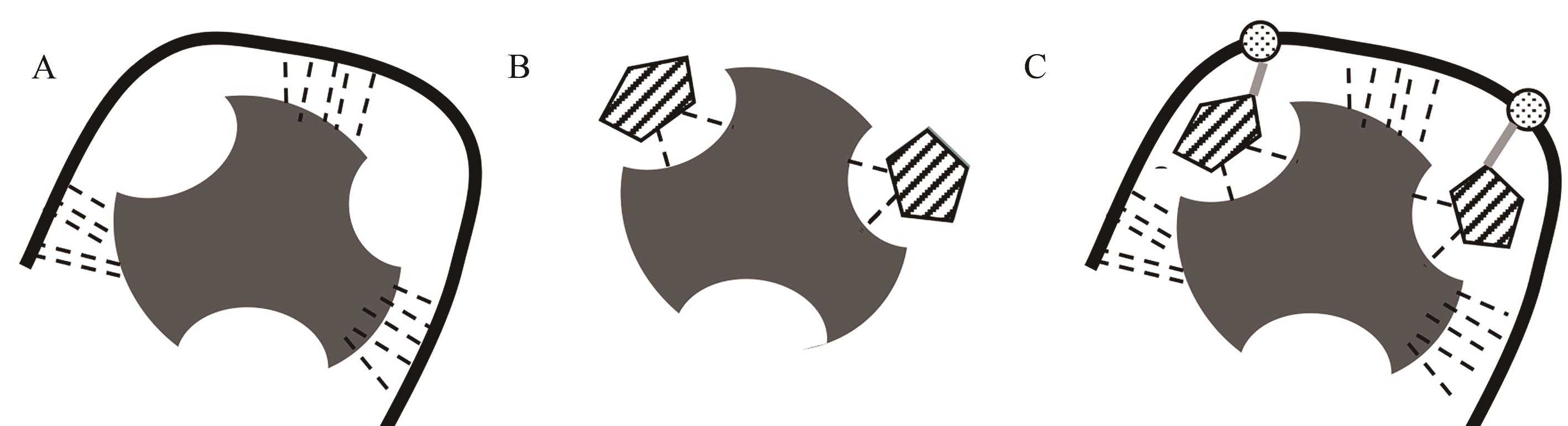
Fig. 3 Different substances bind specifically to lectinsA:Specific binding of aptamer-lectin; B:Specific binding of sugar-lectin; C:Specific binding non natural aptamer-lectin
传感器类型 Sensor type | 具体方法 Concrete method | 检测限 Limit of detection | 线性范围 Linearity range | 文献 Reference |
|---|---|---|---|---|
| 电化学发光 ECL | 石墨烯量子点 GQDs | 0.16 pg·mL-1 | 0.5 pg·mL-1~1.0 ng·mL-1 | [ |
| 电化学发光 ECL | 牛血清白蛋白-富勒烯/铂-钛-苝四羧酸二酐复合材料 BSA-C60/Pt-TiO2-PTC composite | 1.81×10-5 ng·mL-1 | 1.0×10-4~1.0×103 ng·mL-1 | [ |
| 表面等离子体共振 LSPR | 金纳米团簇 AuNCs | 2 nmol·L-1 | 10 nmol·L-1~10 μmol·L-1 | [ |
| 荧光 Fluorescence | 硼酸修饰碳点和荧光微球 BCDs and FMs | 0.089 μg·mL-1 | 0.125~12.500 μg·mL-1 | [ |
| 光电化学 Photoelectrochemistry | 硫化银和溴氧化铋复合材料 Ag2S and BiOBr oxide composite | 0.35 pg·mL-1 | 1.0×10-3~1.0×103 ng·mL-1 | [ |
| 电化学发光 ECL | 多壁碳纳米管配合物 Multi-walled carbon nanotube complexes | 0.3 pg·mL-1 | 0.5 pg·mL-1~100.0 ng·mL-1 | [ |
| 荧光 Fluorescence | 金纳米团簇 AuNCs | 0.62 nmol·L-1 | 0.01~1.00 μmol·L-1 | [ |
| 电化学发光 ECL | 氮化硼量子点 BNQDs | 0.15 pg·mL-1 | 1.0 pg·mL-1~1.0 μg·mL-1 | [ |
| 离子敏感的场效应晶体管 ISFET | 银纳米颗粒 AgNPs | 0.16 ng·mL-1 | — | [ |
| 电化学发光 ECL | 金纳米颗粒 AuNPs | 0.146 µg·mL-1 | 0.19~10.00 µg·mL-1 | [ |
| 场效应晶体管 FET | 金纳米颗粒 AuNPs | 105 nmol·L-1 | — | [ |
Table 1 Comparison of different detection methods for Con A
传感器类型 Sensor type | 具体方法 Concrete method | 检测限 Limit of detection | 线性范围 Linearity range | 文献 Reference |
|---|---|---|---|---|
| 电化学发光 ECL | 石墨烯量子点 GQDs | 0.16 pg·mL-1 | 0.5 pg·mL-1~1.0 ng·mL-1 | [ |
| 电化学发光 ECL | 牛血清白蛋白-富勒烯/铂-钛-苝四羧酸二酐复合材料 BSA-C60/Pt-TiO2-PTC composite | 1.81×10-5 ng·mL-1 | 1.0×10-4~1.0×103 ng·mL-1 | [ |
| 表面等离子体共振 LSPR | 金纳米团簇 AuNCs | 2 nmol·L-1 | 10 nmol·L-1~10 μmol·L-1 | [ |
| 荧光 Fluorescence | 硼酸修饰碳点和荧光微球 BCDs and FMs | 0.089 μg·mL-1 | 0.125~12.500 μg·mL-1 | [ |
| 光电化学 Photoelectrochemistry | 硫化银和溴氧化铋复合材料 Ag2S and BiOBr oxide composite | 0.35 pg·mL-1 | 1.0×10-3~1.0×103 ng·mL-1 | [ |
| 电化学发光 ECL | 多壁碳纳米管配合物 Multi-walled carbon nanotube complexes | 0.3 pg·mL-1 | 0.5 pg·mL-1~100.0 ng·mL-1 | [ |
| 荧光 Fluorescence | 金纳米团簇 AuNCs | 0.62 nmol·L-1 | 0.01~1.00 μmol·L-1 | [ |
| 电化学发光 ECL | 氮化硼量子点 BNQDs | 0.15 pg·mL-1 | 1.0 pg·mL-1~1.0 μg·mL-1 | [ |
| 离子敏感的场效应晶体管 ISFET | 银纳米颗粒 AgNPs | 0.16 ng·mL-1 | — | [ |
| 电化学发光 ECL | 金纳米颗粒 AuNPs | 0.146 µg·mL-1 | 0.19~10.00 µg·mL-1 | [ |
| 场效应晶体管 FET | 金纳米颗粒 AuNPs | 105 nmol·L-1 | — | [ |
检测技术 Measurement technique | 灵敏度 Sensitivity | 设备需求 Demand for equipment | 检测成本 Inspection cost | 通量 Flux | 操作 Operation | 速度 Speed | 特异性 Specificity |
|---|---|---|---|---|---|---|---|
质谱、色谱等高ECL精度检测设备 MS, chromatography and other high precision detection equipment | 高 High | 高 High | 高 High | 低 Low | 高 High | 慢 Slow | 高 High |
血凝法 Hemagglutination method | 中等 Medium | 低 Low | 中等 Medium | 高 High | 低 Low | 快 Fast | 低 Low |
免疫法 Immunoassay method | 中等 Medium | 中等 Medium | 中等 Medium | 高 High | 中等 Medium | 中等 Medium | 中等 Medium |
基于纳米材料的生物传感技术 Nanomaterial based biosensing technology | 高 High | 高 High | 高 High | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 低 Low |
免疫传感技术 Immunosensing technology | 高 High | 中等 Medium | 高 High | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 中等 Medium |
糖传感技术 Sugar sensing technology | 高 High | 中等 Medium | 中等 Medium | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 高,但是不能区分糖特异性相同的凝集素 High, but not distinguishable from lectins with the same sugar specificity |
核酸适配体传感技术 Aptamer sensing technology | 高 High | 中等 Medium | 中等 Medium | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 中等,非天然核酸适配体高 Medium,non-natural aptamers are high |
Table 2 Comparison of different detection techniques for Con A
检测技术 Measurement technique | 灵敏度 Sensitivity | 设备需求 Demand for equipment | 检测成本 Inspection cost | 通量 Flux | 操作 Operation | 速度 Speed | 特异性 Specificity |
|---|---|---|---|---|---|---|---|
质谱、色谱等高ECL精度检测设备 MS, chromatography and other high precision detection equipment | 高 High | 高 High | 高 High | 低 Low | 高 High | 慢 Slow | 高 High |
血凝法 Hemagglutination method | 中等 Medium | 低 Low | 中等 Medium | 高 High | 低 Low | 快 Fast | 低 Low |
免疫法 Immunoassay method | 中等 Medium | 中等 Medium | 中等 Medium | 高 High | 中等 Medium | 中等 Medium | 中等 Medium |
基于纳米材料的生物传感技术 Nanomaterial based biosensing technology | 高 High | 高 High | 高 High | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 低 Low |
免疫传感技术 Immunosensing technology | 高 High | 中等 Medium | 高 High | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 中等 Medium |
糖传感技术 Sugar sensing technology | 高 High | 中等 Medium | 中等 Medium | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 高,但是不能区分糖特异性相同的凝集素 High, but not distinguishable from lectins with the same sugar specificity |
核酸适配体传感技术 Aptamer sensing technology | 高 High | 中等 Medium | 中等 Medium | 若与微阵列结合具有高通量检测的潜质 It has the potential of high throughput detection when combined with microarray | 中等 Medium | 快 Fast | 中等,非天然核酸适配体高 Medium,non-natural aptamers are high |
| 1 | TSANEVA M, VAN DAMME E J M. 130 years of plant lectin research [J]. Glycoconj. J., 2020, 37(5): 533-551. |
| 2 | NAITHANI S, KOMATH S S, NONOMURA A, et al.. Plant lectins and their many roles: carbohydrate-binding and beyond [J/OL]. J. Plant Physiol., 2021, 266:153531 [2023-01-16]. . |
| 3 | AHIRWAR R, NAHAR P. Screening and identification of a DNA aptamer to concanavalin A and its application in food analysis [J]. J. Agric. Food Chem., 2015, 63(16): 4104-4111. |
| 4 | SUMNER J B, HOWELL S F. The isolation of a fourth crystallizable jack bean globulin through the digestion of canavalin with trypsin [J]. J. Biol. Chem., 1936, 113(3): 607-610. |
| 5 | CAVADA B S, OSTERNE V J S, LOSSIO C F, et al.. One century of ConA and 40 years of ConBr research: a structural review [J]. Int. J. Biol. Macromol., 2019, 134: 901-911. |
| 6 | PAN L, YUAN Z, FAROUK M H, et al.. Isolation and analysation of soybean agglutinin-specific binding proteins for erythrocyte membrane in different animal species [J]. Ital. J. Anim. Sci., 2021, 20(1): 84-93. |
| 7 | KUMAR S, VERMA A K, DAS M, et al.. Molecular mechanisms of IgE mediated food allergy [J]. Int. Immunopharmacol., 2012, 13(4): 432-439. |
| 8 | KUMAR S, VERMA A K, DAS M, et al.. Clinical complications of kidney bean (Phaseolus vulgaris L.) consumption [J]. Nutrition, 2013, 29(6): 821-827. |
| 9 | MIYAKE K, TANAKA T, MCNEIL P L. Lectin-based food poisoning: a new mechanism of protein toxicity [J/OL]. PLoS One, 2007, 2(8): e687 [2023-01-16]. . |
| 10 | NONIS S G, HAYWOOD J, SCHMIDBERGER J W, et al.. Structural and biochemical analyses of concanavalin A circular permutation by jack bean asparaginyl endopeptidase [J]. Plant Cell, 2021, 33(8): 2794-2811. |
| 11 | JANG H, LEE C, HWANG Y, et al.. Concanavalin A: coordination diversity to xenobiotic metal ions and biological consequences [J]. Dalton Trans., 2021, 50(48): 17817-17831. |
| 12 | GERLITS O O, COATES L, WOODS R J, et al.. Mannobiose binding induces changes in hydrogen bonding and protonation states of acidic residues in concanavalin A as revealed by neutron crystallography [J]. Biochemistry, 2017, 56(36): 4747-4750. |
| 13 | HULDANI H, RASHID A I, TURAEV K N, et al.. Concanavalin A as a promising lectin-based anti-cancer agent: the molecular mechanisms and therapeutic potential [J/OL]. Cell Commun. Signal., 2022, 20(1): 167 [2023-01-16]. . |
| 14 | JIANG H R, WEN X X, ZHANG X, et al.. Concanavalin A inhibits human liver cancer cell migration by regulating F-actin redistribution and assembly via MAPK signaling pathway [J/OL]. Oncol. Lett., 2022, 24(5): 405 [2023-01-16]. . |
| 15 | YE T, WANG T, YANG X, et al.. Comparison of concanavalin A-induced murine autoimmune hepatitis models [J]. Cell. Physiol. Biochem., 2018, 46(3): 1241-1251. |
| 16 | HE S, SIMPSON B K, SUN H, et al.. Phaseolus vulgaris lectins: a systematic review of characteristics and health implications [J]. Crit. Rev. Food Sci. Nutr., 2018, 58(1): 70-83. |
| 17 | 代素娥, 魏科, 邓鹏, 等. 一起食用未成熟刀豆引起食物中毒的调查分析[J]. 医学动物防制, 2022, 38(4): 402-404, 408. |
| DAI S E, WEI K, DENG P, et al.. An investigation and analysis on food poisoning caused by consuming immature concanavalin [J]. J. Med. Pest Control, 2022, 38(4): 402-404, 408. | |
| 18 | 任三国, 陈高, 郭瑞, 等. 121份菜豆品种资源的植物凝集素含量分析[J]. 江汉大学学报(自然科学版), 2016, 44(6): 504-508. |
| REN S G, CHEN G, GUO R, et al.. Lectin content analysis of 121 common bean cultivars [J]. J. Jianghan Univ. (Nat. Sci. ), 2016, 44(6): 504-508. | |
| 19 | 任三国, 陈高, 郭瑞, 等. 菜豆种质资源的植物凝集素含量测定与评价[J]. 长江蔬菜, 2017 (16): 36-38. |
| REN S G, CHEN G, GUO R, et al.. Determination and evaluation of lectin contents in kidney bean (Phaseolus vulgaris L.) germplasm resources [J]. J. Changjiang Veget., 2017 (16): 36-38. | |
| 20 | 胡燕秋, 冯国军, 杨晓旭, 等. 菜豆种质资源的植物凝集素含量测定及变化分析[J]. 中国农学通报, 2020, 36(5): 18-25. |
| HU Y Q, FENG G J, YANG X X, et al.. Phytohemagglutinin in common bean germplasm resources: determination and content variation analysis [J]. Chin. Agric. Sci. Bull., 2020, 36(5): 18-25. | |
| 21 | AHIRWAR R, NAHAR P. Development of an aptamer-affinity chromatography for efficient single step purification of concanavalin A from Canavalia ensiformis [J]. J. Chromatogr. B, 2015, 997: 105-109. |
| 22 | WEN Y, LIU A G, MENG C Z, et al.. Quantification of lectin in soybeans and soy products by liquid chromatography-tandem mass spectrometry [J/OL]. J. Chromatogr. B, 2021, 1185: 122987 [2023-01-16]. . |
| 23 | HE S, SHI J, LI X, et al.. Identification of a lectin protein from black turtle bean (Phaseolus vulgaris) using LC-MS/MS and PCR method [J]. LWT-Food Sci. Technol., 2015, 60(2): 1074-1079. |
| 24 | PURKAIT S, KOLEY S. Identification and characterization of lectins from leguminosae plants [J]. Int. J. Health Sci. Res., 2019, 9(2): 115-121. |
| 25 | HAMED E S E, IBRAHIM E A M M, MOUNIR S M. Antimicrobial activities of lectins extracted from some cultivars of phaseolus vulgaris seeds [J]. J. Microb. Biochem. Technol., 2017, 9(3): 109-116. |
| 26 | 冷艳, 孙宪迅, 王璐, 等. 植物凝集素检测方法的研究进展[J]. 江汉大学学报(自然科学版), 2018, 46(4): 305-309. |
| LENG Y, SUN X X, WANG L, et al.. Research progress of detection methods of plant lectin [J]. J. Jianghan Univ. (Nat. Sci. ), 2018, 46(4): 305-309. | |
| 27 | ZHAO J, HE S, TANG M, et al.. Low-pH induced structural changes, allergenicity and in vitro digestibility of lectin from black turtle bean (Phaseolus vulgaris L.) [J]. Food Chem., 2019, 283: 183-190. |
| 28 | 湛润生, 戎婷婷, 白静. 血凝法测定绿豆与红小豆中植物凝集素质量分数[J]. 食品科技, 2017, 42(4): 277-279. |
| ZHAN R S, RONG T T, BAI J. Quantification of lectins in mung bean and red adzuki bean by hemagglutination [J]. Food Sci. Technol., 2017, 42(4): 277-279. | |
| 29 | VINCENZI S, ZOCCATELLI G, PERBELLINI F, et al.. Quantitative determination of dietary lectin activities by enzyme-linked immunosorbent assay using specific glycoproteins immobilized on microtiter plates [J]. J. Agric. Food. Chem., 2002, 50(22): 6266-6270. |
| 30 | 许键, 朱兵, 杨金菊, 等. 大豆针刺后SBA定量方法的研究[J]. 八一农学院学报, 1993,16 (3): 25-27. |
| XU J, ZHU B, YANG J J, et al.. Study on the SBA quantitating post acupuncture [J]. J. August 1st Agric. Coll., 1993, 16(3): 25-27. | |
| 31 | 杨丽杰, 李素芬, 张永成, 等. 黑龙江几个大豆品种中抗营养因子含量的分析[J]. 大豆科学, 1999, 18(1): 78-81. |
| YANG L J, LI S F, ZHANG Y C, et al.. The content of main antinutritional factors in thirteen soybean varieties planted widely in Heilongjiang area [J]. Soybean Sci., 1999, 18(1): 78-81. | |
| 32 | 张柏林, 秦贵信, 刘宁, 等. 大豆凝集素结构及其活性测定方法的研究进展[J]. 大豆科学, 2009, 28(1): 160-163. |
| ZHANG B L, QIN G X, LIU N, et al.. Advances of research on structure and activity detecting method of soybean agglutinin [J]. Soybean Sci., 2009, 28(1): 160-163. | |
| 33 | ZUO F, ZHANG C, ZHANG H, et al.. A solid-state electrochemiluminescence biosensor for ConA detection based on CeO2@Ag nanoparticles modified graphene quantum dots as signal probe [J]. Electrochim. Acta, 2019, 294: 76-83. |
| 34 | ABAD-GIL L, GISMERA M J, SEVILLA M T, et al.. Methylisothiazolinone response on disposable electrochemical platforms modified with carbon, nickel or gold-based nanomaterials [J/OL]. Electrochim. Acta, 2020, 187(4): 199[2023-01-16]. . |
| 35 | GAO X, WANG L, SUN C, et al.. Research on preparation methods of carbon nanomaterials based on self-assembly of carbon quantum dots [J/OL]. Molecules, 2022, 27(5): 1690 [2023-01-16]. . |
| 36 | NARAYANAN K B, SAKTHIVEL N, HAN S S. From chemistry to biology: applications and advantages of green, biosynthesized/biofabricated metal-and carbon-based nanoparticles [J]. Fibers Polym., 2021, 22(4): 877-897. |
| 37 | 陆瑶. 基于有机复合材料的电化学发光生物传感器的研制及应用[D].上海:上海师范大学, 2021. |
| LU Y. Development and application of electrochemical luminescence biosensors based on organic composites for medical animal control [D]. Shanghai: Shanghai Normal University, 2021. | |
| 38 | SHEN J, ZHANG L, LIU L, et al.. Revealing lectin-sugar interactions with a single Au@Ag nanocube [J]. ACS Appl. Mater. Interfaces, 2019, 11(43): 40944-40950. |
| 39 | 王宇菲. 基于荧光微球的生物传感方法用于生物标志物的检测[D]. 郑州:郑州大学, 2021. |
| WANG Y F. Biosensing methods based on fluorescent microspheres are used for the detection of biomarkers [D]. Zhengzhou: Zhengzhou University, 2021. | |
| 40 | 陈代武, 彭若君, 李核. 基于Ag2S/BiOBr的传感器检测人体血清中刀豆球蛋白A的研究[J].邵阳学院学报(自然科学版), 2022, 19(4): 51-58. |
| CHEN D W, PENG R J, LI H. Detection of concanavalin A in human serum by Ag2S/BiOBr sensor [J]. J. Shaoyang Univ. (Nat. Sci. ), 2022, 19(4): 51-58. | |
| 41 | GOODARZI M T, TURNER G A. A lectin method for investigating the glycosylation of nanogram amounts of purified glycoprotein [J]. Glycoconj. J., 1997, 14(4): 493-496. |
| 42 | SUN X, YE Y, HE S, et al.. A novel oriented antibody immobilization based voltammetric immunosensor for allergenic activity detection of lectin in kidney bean by using AuNPs-PEI-MWCNTs modified electrode [J/OL]. Biosens. Bioelectron., 2019, 143: 111607 [2023-01-16]. . |
| 43 | AFKHAMI A, HASHEMI P, BAGHERI H, et al.. Impedimetric immunosensor for the label-free and direct detection of botulinum neurotoxin serotype A using Au nanoparticles/graphene-chitosan composite [J]. Biosens. Bioelectron., 2017, 93: 124-131. |
| 44 | TANG T, YANG F, WANG L, et al.. A sandwich electrochemiluminescent assay for determination of concanavalin A with triple signal amplification based on MoS2 NF@ MWCNTs modified electrode and Zn-MOF encapsulated luminol [J/OL]. Microchim. Acta, 2020, 187(9): 523 [2023-01-16]. . |
| 45 | SHA Q, GUAN R, SU H, et al.. Carbohydrate-protein template synthesized high mannose loading gold nanoclusters: a powerful fluorescence probe for sensitive concanavalin A detection and specific breast cancer cell imaging [J/OL]. Talanta, 2020, 218: 121130 [2023-01-16]. . |
| 46 | WANG C X, LI M S, WANG P J, et al.. An electrochemiluminescence biosensor based on boron nitride quantum dots as novel coreactant for quantitative determination of concanavalin A [J]. Microchim. Acta, 2020, 187(7): 409 [2023-01-16]. . |
| 47 | ZHAO S, SHI C, HU H, et al.. ISFET and Dex-AgNPs based portable sensor for reusable and real-time determinations of concanavalin A and glucose on smartphone [J/OL]. Biosens. Bioelectron., 2020, 151: 111962 [2023-01-16]. . |
| 48 | SHA H, ZHANG Y, WANG Y, et al.. Electrochemiluminescence resonance energy transfer biosensor between the glucose functionalized MnO2 and g-C3N4 nanocomposites for ultrasensitive detection of concanavalin A [J]. Biosens. Bioelectron., 2019, 124: 59-65. |
| 49 | SUN C, SHEN Y, ZHANG Y, et al.. Sandwich photoelectrochemical biosensing of concanavalin A based on CdS/AuNPs/NiO Z-scheme heterojunction and lectin-sugar binding [J/OL]. Talanta, 2023, 253: 123882 [2023-01-16]. . |
| 50 | KWON J, AHN K S, JEONG D, et al.. Highly sensitive determination of concanavalin A lectin based on silver-enhanced electrogenerated chemiluminescence of luminol [J]. Anal. Lett., 2018, 51(13): 2114-2127. |
| 51 | MA M, CHAO L, ZHAO Y, et al.. High-sensitivity detection of concanavalin A using MoS2-based field effect transistor biosensor [J/OL]. J. Phys. D, 2021, 54(24): 245401[2023-01-16]. . |
| 52 | ELLINGTON A D, SZOSTAK J W. In vitro selection of RNA molecules that bind specific ligands [J]. Nature, 1990, 346(6287): 818-822. |
| 53 | TUERK C, GOLD L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase [J]. Science, 1990, 249(4968): 505-510. |
| 54 | GUAN B, ZHANG X. Aptamers as versatile ligands for biomedical and pharmaceutical applications [J]. Int. J. Nanomed., 2020, 15: 1059-1071. |
| 55 | CHEN W, CUI L, LI C, et al.. A novel aptamer biosensor using ZnO-3DNGH for sensitive and selective detection of Listeria monocytogenes [J/OL]. Microchem. J., 2022, 179: 107414 [2023-01-16]. . |
| 56 | ZHIPENG S, LIYONG H, YUTING C, et al.. Multiplexed electrochemical aptasensor for antibiotics detection using metallic-encoded apoferritin probes and double stirring bars-assisted target recycling for signal amplification [J]. Talanta, 2019, 197: 491-499. |
| 57 | SONG K M, LEE S, BAN C. Aptamers and their biological applications [J]. Sensors, 2012, 12(1): 612-631. |
| 58 | SARKAR N, SHARMA R S, KAUSHIK M. Exploring the potential of DNA/RNA aptamers in national security [J]. Natl. Acad. Sci. Lett., 2020,43(2): 187-190. |
| 59 | LIU H, BAI Y, QIN J, et al.. A novel fluorescent concanavalin A detection platform using an anti-concanavalin A aptamer and graphene oxide [J]. Anal. Methods, 2017, 9(5): 744-747. |
| 60 | GORDON C K L, WU D, PUSULURI A, et al.. Click-particle display for base-modified aptamer discovery [J]. ACS Chem. Biol., 2019, 14(12): 2652-2662. |
| [1] | Guanqun CHAI, Li WANG, Guihua LIU, Xinjian LUOMU, Ya JIANG, Hong LIANG, Chengwu FAN. Health Risk Assessment of Heavy Metals and Differences in Cd Uptake and Accumulation of Three Types of Pod Peppers [J]. Journal of Agricultural Science and Technology, 2023, 25(3): 169-177. |
| [2] | LI Pei-wu, DING Xiao-xia. Studies on Control Technology for Grain and Oil Quality and Safety in China and Development Countermeasure [J]. , 2011, 13(5): 54-58. |
| [3] | WANG Xiao-Yu, ZHOU Zun-Chun, GUAN Xiao-yan, JIANG Bei, CHEN Zhong, DONG Ying, YA. Rapid PCR Detection for Vibrio alginolyticus and Vibrio splendidus in Sea Cucumber Apostichopus japonicas and its Culturing Environment [J]. , 2010, 12(3): 125-130. |
| [4] | MA Yan1|2|LI Jian2|WANG Qun2|HE Yu-ying2|WANG Bin1. Rapid and Semi-quantitative Detection of Pathogenic Vibrio paraharmolyticus from Environment and Aquatic Animals by PC-PCR [J]. , 2008, 10(S1): 67-72. |
| [5] | ZHOU Wei, FAN Zhi-hong, CAO Zhan, LIU Fang . Health Care Function of Anti-nutritional Factors in Legumes [J]. , 2007, 9(4): 61-65. |
| [6] | WENG Bo-qi, WANG Yi-xiang, YING Zhao-yang, HUANG Yi-bin, HUANG Qinlou . Fertilization and the Growth of Legumes in Red Soil Mountains [J]. , 2005, 7(5): 46-49. |
| [7] | ZHAO Zhong-lin1, MA Xin2, LI Yan3, LI Shu-ying4, YUAN Chao1. Intein-based Biosensor [J]. , 1, 1(1): 56-60. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号