




















Journal of Agricultural Science and Technology ›› 2023, Vol. 25 ›› Issue (3): 78-95.DOI: 10.13304/j.nykjdb.2021.0368
• BIOTECHNOLOGY & LIFE SCIENCE • Previous Articles Next Articles
Ye ZHANG( ), Hao ZHANG, Pengpeng ZHANG(
), Hao ZHANG, Pengpeng ZHANG( )
)
Received:2021-04-06
Accepted:2021-06-01
Online:2023-03-15
Published:2023-05-22
Contact:
Ye ZHANG,Pengpeng ZHANG
Supported by:通讯作者:
张叶,张芃芃
CLC Number:
Ye ZHANG, Hao ZHANG, Pengpeng ZHANG. Interplay of Cyclic Electron Transport and Mehler-like Reaction in Synechocystis Under Different Light Regimes[J]. Journal of Agricultural Science and Technology, 2023, 25(3): 78-95.
张叶, 张昊, 张芃芃. 不同光环境集胞藻循环电子传递与类梅勒反应[J]. 中国农业科技导报, 2023, 25(3): 78-95.
| Primer | Sequence (5’-3’) |
|---|---|
| pUC-FLV1-up-R | TTGGCAGAATTGCGGAGC |
| pUC-FLV1-up-F-BamHI | CGCGGATCCACTAACCCTGCGGCTCGG |
| pUC-FLV1-down-R-KpnI | CGGGGTACCCCCTGGCTTATTTGGAATGTCGGG |
| pUC-FLV1-down-F-EcoRI | CCGGAATTCCAATTCTGAGCATCGTTTGGTGTA |
| pUC-PGR5-up-R- BamHI | CGCGGATCCGAACTTAGCTTTGCCTAACTGTTGA |
| pUC-PGR5-up-F-HindIII | CCCAAGCTTCGCTCAAAAATGAATGTGGATG |
| pUC-PGR5-down-R-EcoRI | GCCACATCTCGACCCAGTTGA |
| pUC-PGR5-down-F-KpnI | CGGGGTACCCCGAAAGCAGGGATAATAGCC |
| petJ-PstI | AACTGCAGGGG AAT TGC TCT GGC AAC T |
| petJ-SalI-oop-BamHI | CGGGATCCAATAAAAAACGCCCGGCGGCAACCGAGCGGTCGACAGTTCTCCTTTCAAGGATAAAGTC |
| Flv1-F-SalI | AGGCGTCGACGTGGGAATCCATGCAAAAC |
| Flv1-R-His-SalI | AGGCGTCGACCTAATGATGATGATGATGATGATAATGATCGCCAGATTTCC |
| spkA-up-R-PstI | CACTGCAGGCTTCCCGTTCAAACCGT |
| spkA-up-F-PstI | CACTGCAGACATAGGCCGCTTCACCA |
| spkA-down-R-EcoRI | CGGAATTCTGGTCAGTTTCGCCCGAT |
| spkA-down-F-EcoRI | CGGAATTCCAACGCCGCTGAAGGAAA |
| FLV1-S-F | CGAGAAAACGGTGTATTAGGACT |
| FLV1-S-R | ACTCAGTCAGCGCCAACA |
| PGR5-S-F | ATTTCCAGCGGATCAGGC |
| PGR5-S-R | GCCACATCTCGACCCAGTTGA |
| qsll1521-F | AGGGCCTTTGACTTGAAGCG |
| qsll1521-R | CAGTCAGGGACCATAGCACAGG |
| qrnpB-F | GGAGGGGGCAATGAAGTT |
| qrnpB-R | GGCGTTACCCAGCAAGTT |
Table 1 Primers in this assay
| Primer | Sequence (5’-3’) |
|---|---|
| pUC-FLV1-up-R | TTGGCAGAATTGCGGAGC |
| pUC-FLV1-up-F-BamHI | CGCGGATCCACTAACCCTGCGGCTCGG |
| pUC-FLV1-down-R-KpnI | CGGGGTACCCCCTGGCTTATTTGGAATGTCGGG |
| pUC-FLV1-down-F-EcoRI | CCGGAATTCCAATTCTGAGCATCGTTTGGTGTA |
| pUC-PGR5-up-R- BamHI | CGCGGATCCGAACTTAGCTTTGCCTAACTGTTGA |
| pUC-PGR5-up-F-HindIII | CCCAAGCTTCGCTCAAAAATGAATGTGGATG |
| pUC-PGR5-down-R-EcoRI | GCCACATCTCGACCCAGTTGA |
| pUC-PGR5-down-F-KpnI | CGGGGTACCCCGAAAGCAGGGATAATAGCC |
| petJ-PstI | AACTGCAGGGG AAT TGC TCT GGC AAC T |
| petJ-SalI-oop-BamHI | CGGGATCCAATAAAAAACGCCCGGCGGCAACCGAGCGGTCGACAGTTCTCCTTTCAAGGATAAAGTC |
| Flv1-F-SalI | AGGCGTCGACGTGGGAATCCATGCAAAAC |
| Flv1-R-His-SalI | AGGCGTCGACCTAATGATGATGATGATGATGATAATGATCGCCAGATTTCC |
| spkA-up-R-PstI | CACTGCAGGCTTCCCGTTCAAACCGT |
| spkA-up-F-PstI | CACTGCAGACATAGGCCGCTTCACCA |
| spkA-down-R-EcoRI | CGGAATTCTGGTCAGTTTCGCCCGAT |
| spkA-down-F-EcoRI | CGGAATTCCAACGCCGCTGAAGGAAA |
| FLV1-S-F | CGAGAAAACGGTGTATTAGGACT |
| FLV1-S-R | ACTCAGTCAGCGCCAACA |
| PGR5-S-F | ATTTCCAGCGGATCAGGC |
| PGR5-S-R | GCCACATCTCGACCCAGTTGA |
| qsll1521-F | AGGGCCTTTGACTTGAAGCG |
| qsll1521-R | CAGTCAGGGACCATAGCACAGG |
| qrnpB-F | GGAGGGGGCAATGAAGTT |
| qrnpB-R | GGCGTTACCCAGCAAGTT |
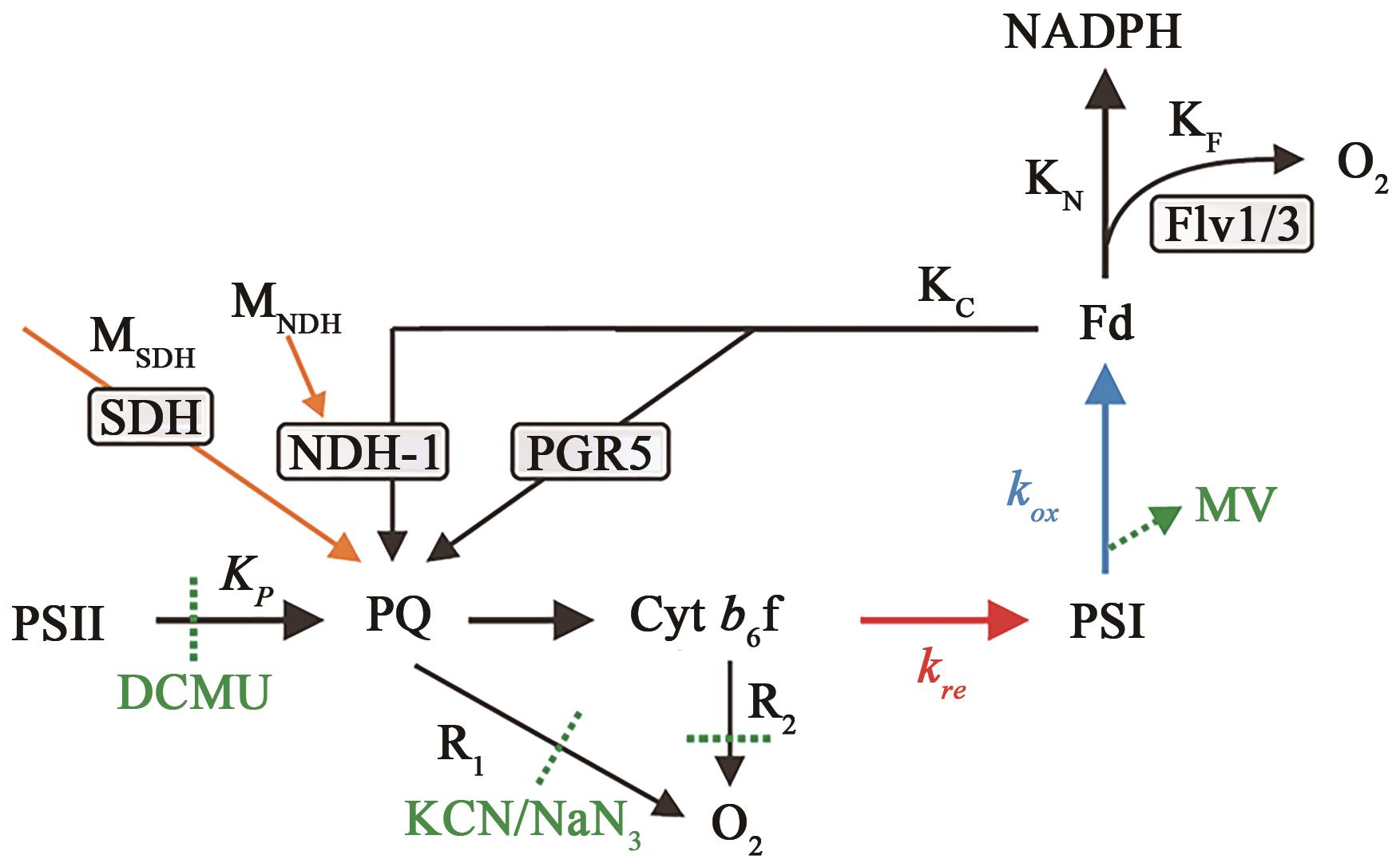
Fig. 1 Major electron transport pathways around PSINote: Dark adapted cells at final chlorophyll concentration 10 μg·mL-1 were illuminated by either orange light (630 nm, 940 μmol photons m-2·s-1) or far-red light (725 nm, 1 400 μmol photons m-2·s-1) for 20 s and followed by darkness. The absorbance changes at 810 nm were monitored by dark-pulse mode using Jts-10 (Biologics). The measurements were performed in the absence or presence of 20 μmol·L-1 DCMU, 200 μmol·L-1 MV or 1 mmol·L-1 NaN3.

Fig. 2 Verification of pgr5 and flv1 mutants by PCRA: Segregation of pgr5 in Δpgr5 and M55/Δpgr5; B: Segregations of flv1 in WT, Δflv1, Δflv1/Δpgr5, flv1/M55 and flv1/M55/Δpgr5
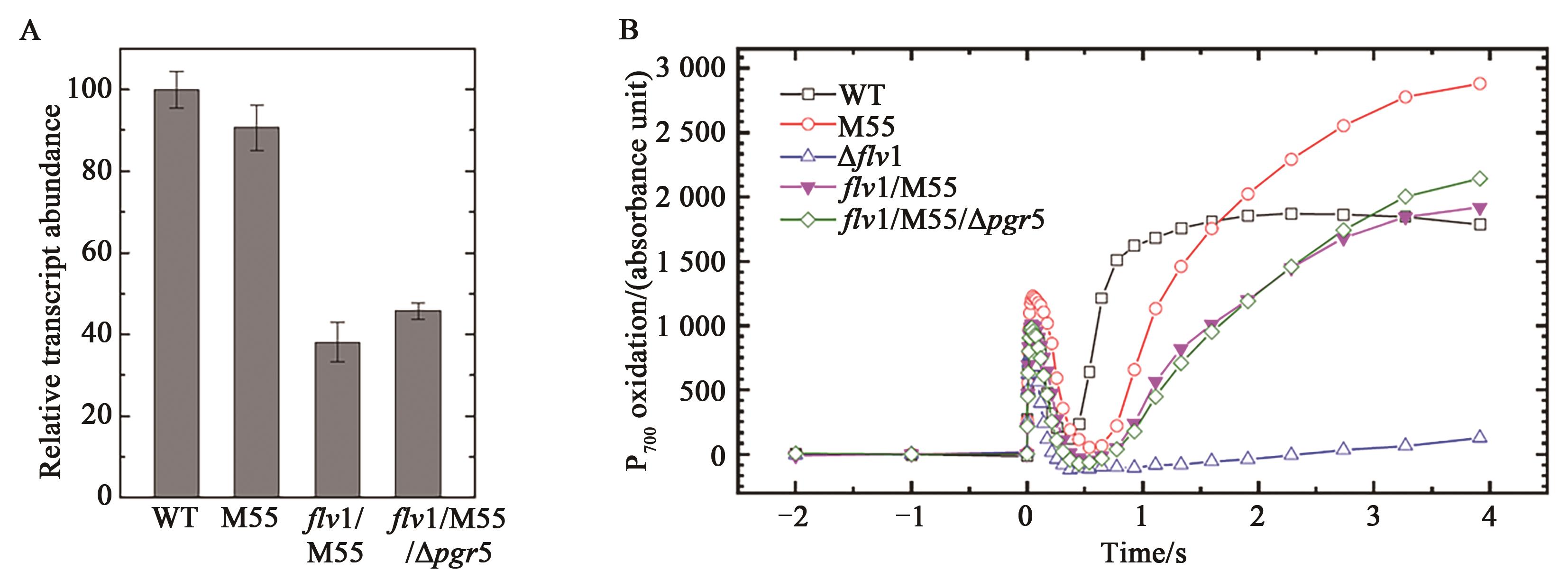
Fig. 3 Characterization of various flv1 mutantsA: Transcript accumulation of flv1 gene in the WT, M55, flv1/M55, and flv1/M55/Δpgr5 analyzed by qRT-PCR, and the result was shown as the average of 3 independent replicates ± SD; B: P700 oxidation kinetics of the WT, Δflv1, M55, flv1/M55, and flv1/M55/Δpgr5. Dark adapted cells were illuminated by 940 μmol photons m-2 s-1 actinic light (630 nm), and the absorbance changes at 810 nm were monitored
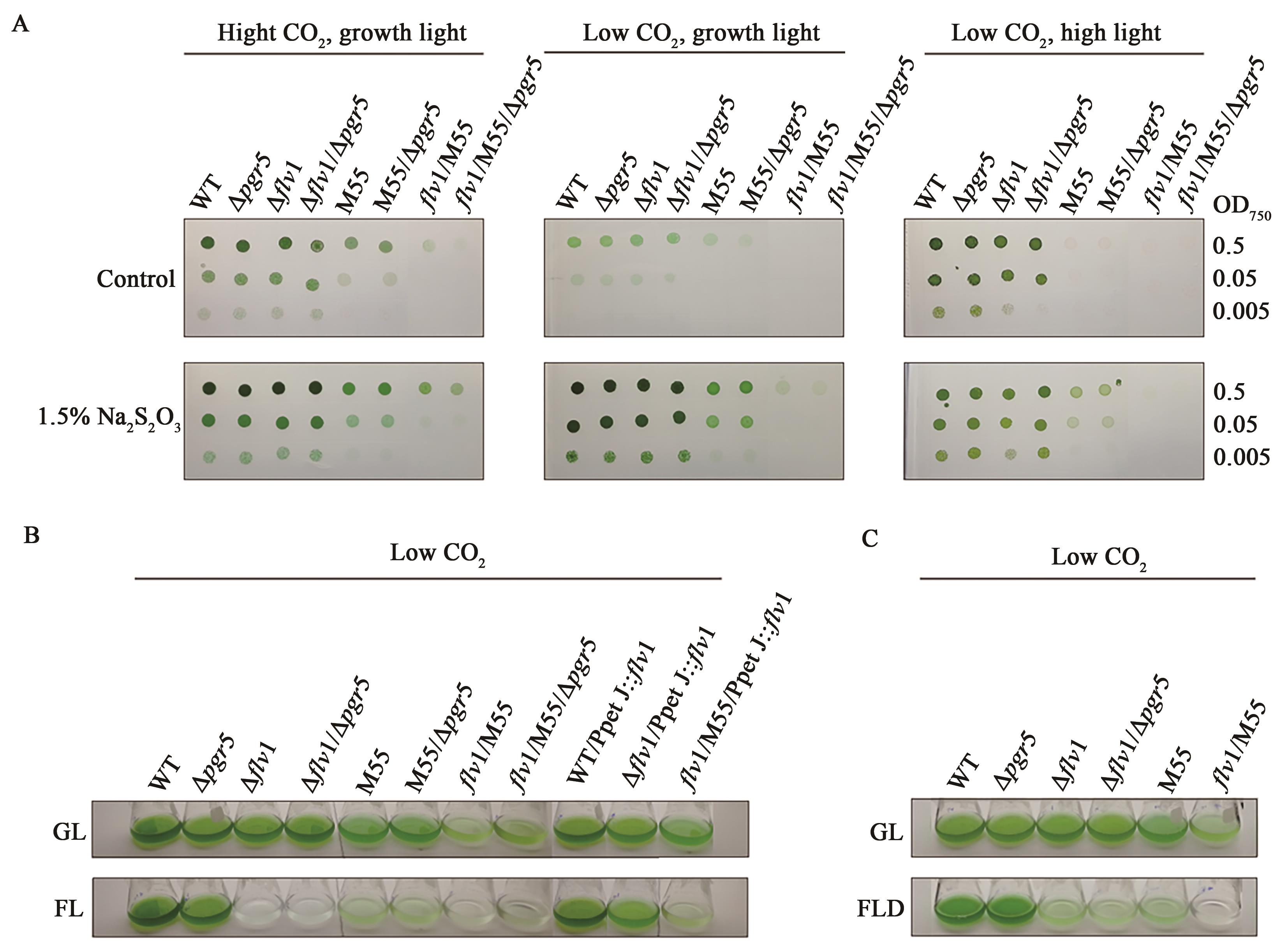
Fig. 4 Growth of WT and mutants under different conditionsA: Growth of WT and various mutants under different CO2 conditions, light intensities and additional Na2S2O3; liquid cells in logarithmic growth were centrifuged and resuspended in fresh BG-11 medium, and diluted to OD750= 0.5, 0.05 and 0.005. 4 μl cell suspension were plated on the agar plate; B: Growth of WT, mutants and overexpression strains under fluctuating light; C: Growth of WT and mutant under combination of fluctuating light and darkness conditions
Fig. 5 Hydrogen peroxide treatment and SOD activities of WT and mutantsA: Growth of WT and mutants in the presence of hydrogen. LC grown cells (0.6-0.8 of OD750) were collected and resuspended in BG-11 medium (pH 8.3) to OD750=0.2. The cultures were supplemented with 1.0, 1.5, 2.0 and 3.0 mmol·L-1 H2O2, respectively, and photographed after 16 and 48 h. B: SOD activity of WT and mutant cells grown under LC and HC conditions. The results are the average of the measurements from three independent cultures
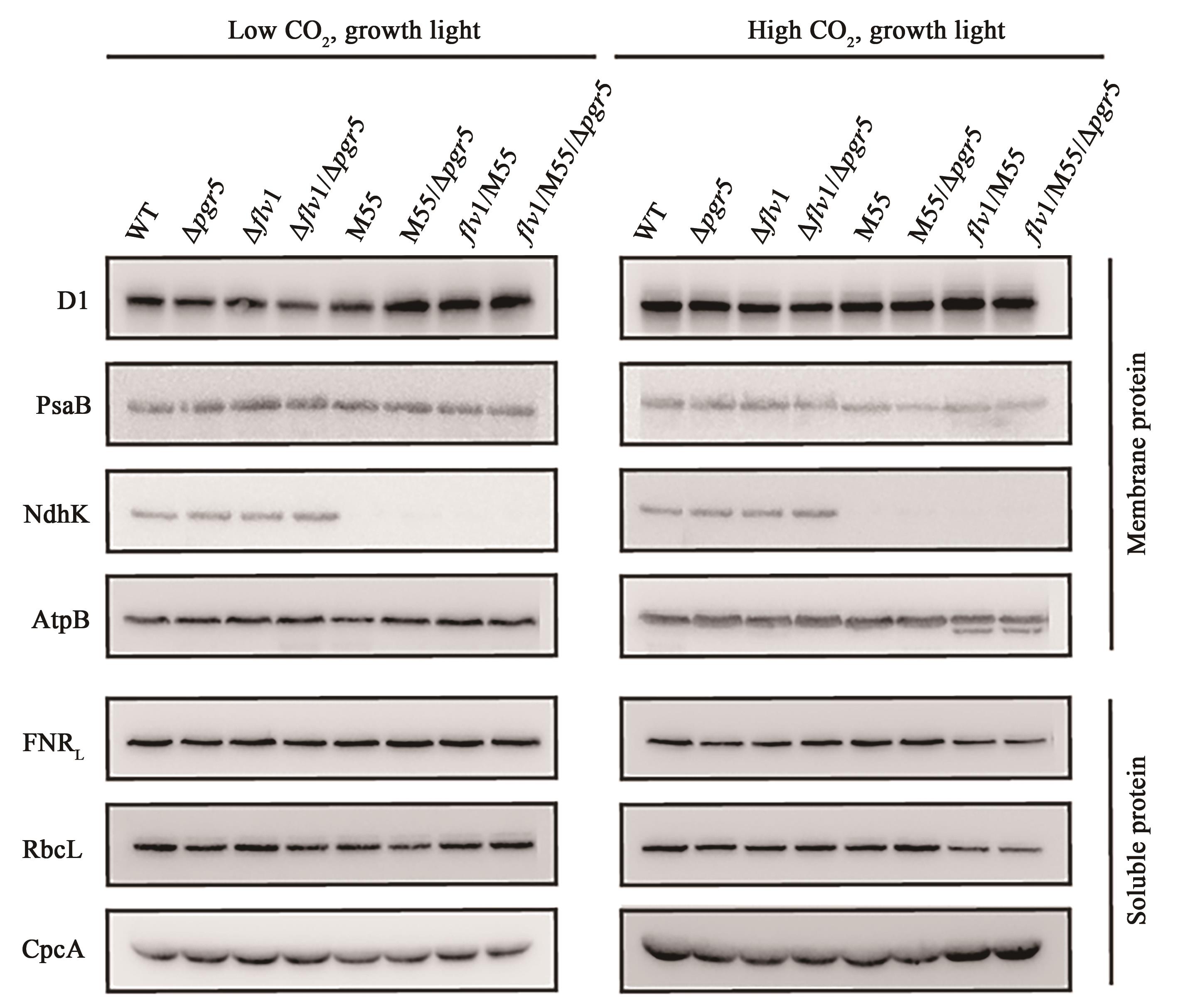
Fig. 6 Expression of major photosynthetic proteins on the thylakoid membranes and in the soluble fractionNote: Membrane or soluble proteins were separated by SDS-PAGE, and immunodetected using specific antibodies against D1, PsaB, NdhK, AtpB, FNR, CpcA and RbcL. In each well, 10 μg protein was loaded.
| Strain | Treatment | D1/% | PsaB/% | PSI/PSII/% | FNRL/% | RbcL/% | CpcA/% |
|---|---|---|---|---|---|---|---|
| WT | LC | 100±5 | 100±5 | 100±5 | 100±6 | 100±5 | 100±1 |
| HC | 183±20 | 112±6 | 61±3 | 113±21 | 96±15 | 100±1 | |
| ∆pgr5 | LC | 94±3 | 106±9 | 114±9 | 90±6 | 87±10 | 137±9 |
| HC | 185±16 | 117±8 | 63±4 | 85±16 | 79±8 | 85±18 | |
| ∆flv1 | LC | 100±18 | 116±23 | 116±23 | 102±1 | 103±2 | 133±18 |
| HC | 180±5 | 118±12 | 66±7 | 81±1 | 82±1 | 67±17 | |
| Δflv1/Δpgr5 | LC | 91±8 | 105±14 | 116±15 | 84±5 | 110±19 | 125±14 |
| HC | 182±16 | 115±1 | 63±1 | 102±5 | 93±1 | 67±10 | |
| M55 | LC | 135±6 | 98±4 | 72±3 | 109±15 | 86±6 | 128±24 |
| HC | 176±2 | 97±5 | 55±3 | 116±6 | 77±9 | 70±6 | |
| M55/∆pgr5 | LC | 163±15 | 94±12 | 58±7 | 110±8 | 89±11 | 140±34 |
| HC | 173±6 | 97±6 | 56±3 | 141±18 | 86±14 | 66±8 | |
| flv1/M55 | LC | 176±14 | 91±17 | 52±9 | 87±19 | 95±22 | 139±17 |
| HC | 179±15 | 84±1 | 47±1 | 67±3 | 25±10 | 101±31 | |
| flv1/M55/∆pgr5 | LC | 164±10 | 90±13 | 55±8 | 96±2 | 99±8 | 104±1 |
| HC | 181±7 | 94±6 | 52±3 | 48±3 | 23±6 | 114±25 |
Table 2 Semi-quantification of D1, PsaB, FNR, RbcL and CpcA
| Strain | Treatment | D1/% | PsaB/% | PSI/PSII/% | FNRL/% | RbcL/% | CpcA/% |
|---|---|---|---|---|---|---|---|
| WT | LC | 100±5 | 100±5 | 100±5 | 100±6 | 100±5 | 100±1 |
| HC | 183±20 | 112±6 | 61±3 | 113±21 | 96±15 | 100±1 | |
| ∆pgr5 | LC | 94±3 | 106±9 | 114±9 | 90±6 | 87±10 | 137±9 |
| HC | 185±16 | 117±8 | 63±4 | 85±16 | 79±8 | 85±18 | |
| ∆flv1 | LC | 100±18 | 116±23 | 116±23 | 102±1 | 103±2 | 133±18 |
| HC | 180±5 | 118±12 | 66±7 | 81±1 | 82±1 | 67±17 | |
| Δflv1/Δpgr5 | LC | 91±8 | 105±14 | 116±15 | 84±5 | 110±19 | 125±14 |
| HC | 182±16 | 115±1 | 63±1 | 102±5 | 93±1 | 67±10 | |
| M55 | LC | 135±6 | 98±4 | 72±3 | 109±15 | 86±6 | 128±24 |
| HC | 176±2 | 97±5 | 55±3 | 116±6 | 77±9 | 70±6 | |
| M55/∆pgr5 | LC | 163±15 | 94±12 | 58±7 | 110±8 | 89±11 | 140±34 |
| HC | 173±6 | 97±6 | 56±3 | 141±18 | 86±14 | 66±8 | |
| flv1/M55 | LC | 176±14 | 91±17 | 52±9 | 87±19 | 95±22 | 139±17 |
| HC | 179±15 | 84±1 | 47±1 | 67±3 | 25±10 | 101±31 | |
| flv1/M55/∆pgr5 | LC | 164±10 | 90±13 | 55±8 | 96±2 | 99±8 | 104±1 |
| HC | 181±7 | 94±6 | 52±3 | 48±3 | 23±6 | 114±25 |

Fig. 7 Kinetic analysis of P700+ reduction in WT and mutantsNote: Kinetics of P700 in WT, Δpgr5, Δflv1, Δflv1/Δpgr5, M55, M55/Δpgr5, flv1/M55, and flv1/M55/Δpgr5. Black square line, 20 μmol·L-1 DCMU; yellow circle line, no addition; blue triangle line, 1 mmol·L-1 NaN3; pink rhombus line, 200 μmol·L-1 MV. The 0.0 and 1.0 on the y axis correspond to fully reduced and oxidized P700, respectively.

Fig. 8 Cyclic electron flow in WT and mutantsA: CET rate determined by P700+ rereduction in WT and mutants under LC and HC; B: CET rate in WT and NDH and PGR5 mutants grown under LC
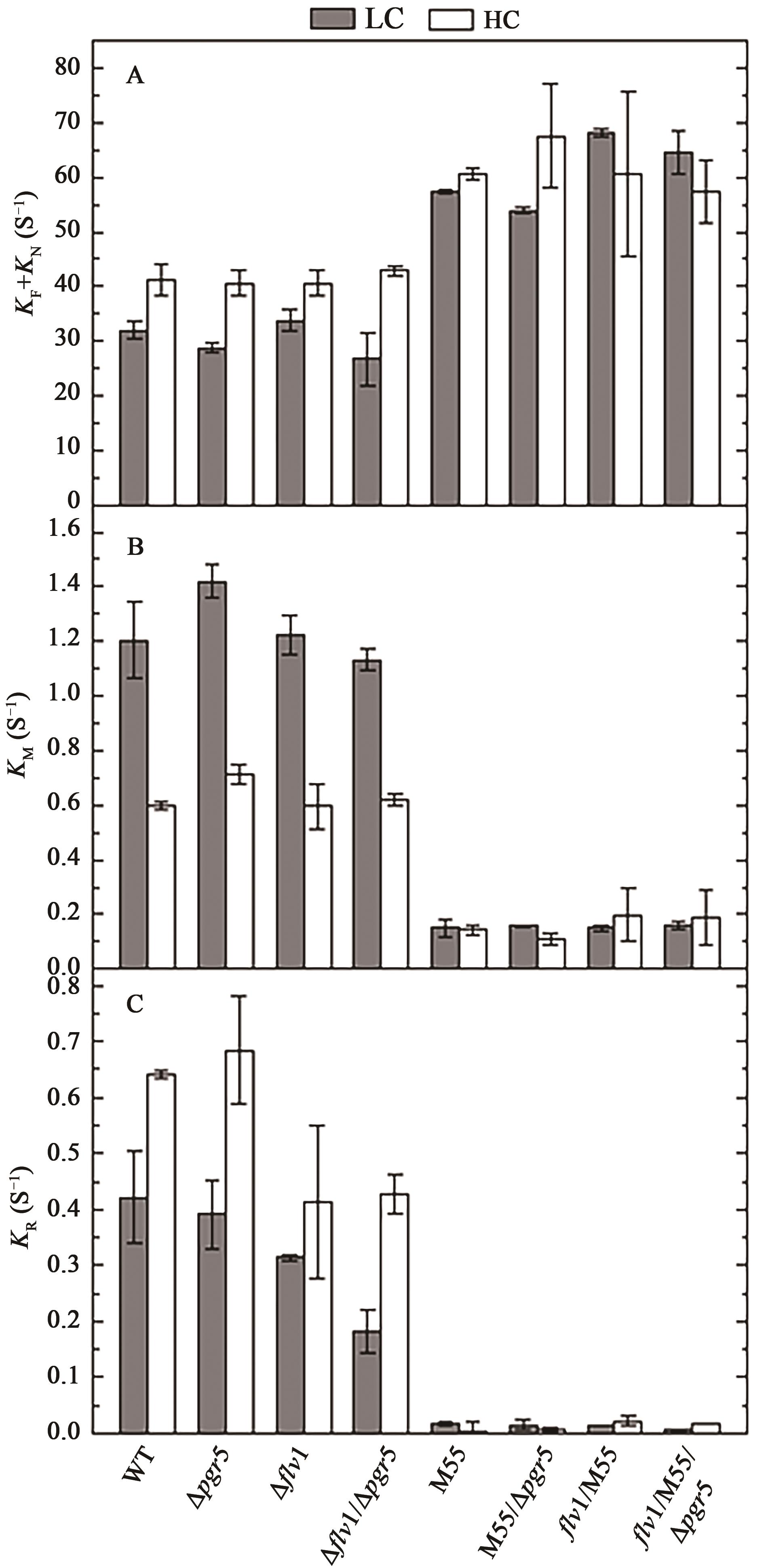
Fig. 9 Electron flows around P700 and PQ pool in WT and mutants under LC and HCA: Electron flows of P700 oxidation via Flv1/3 mediated Mehler-like reaction and FNR; B: Electrons from metabolites; C: Electrons to O2 via terminal oxidases
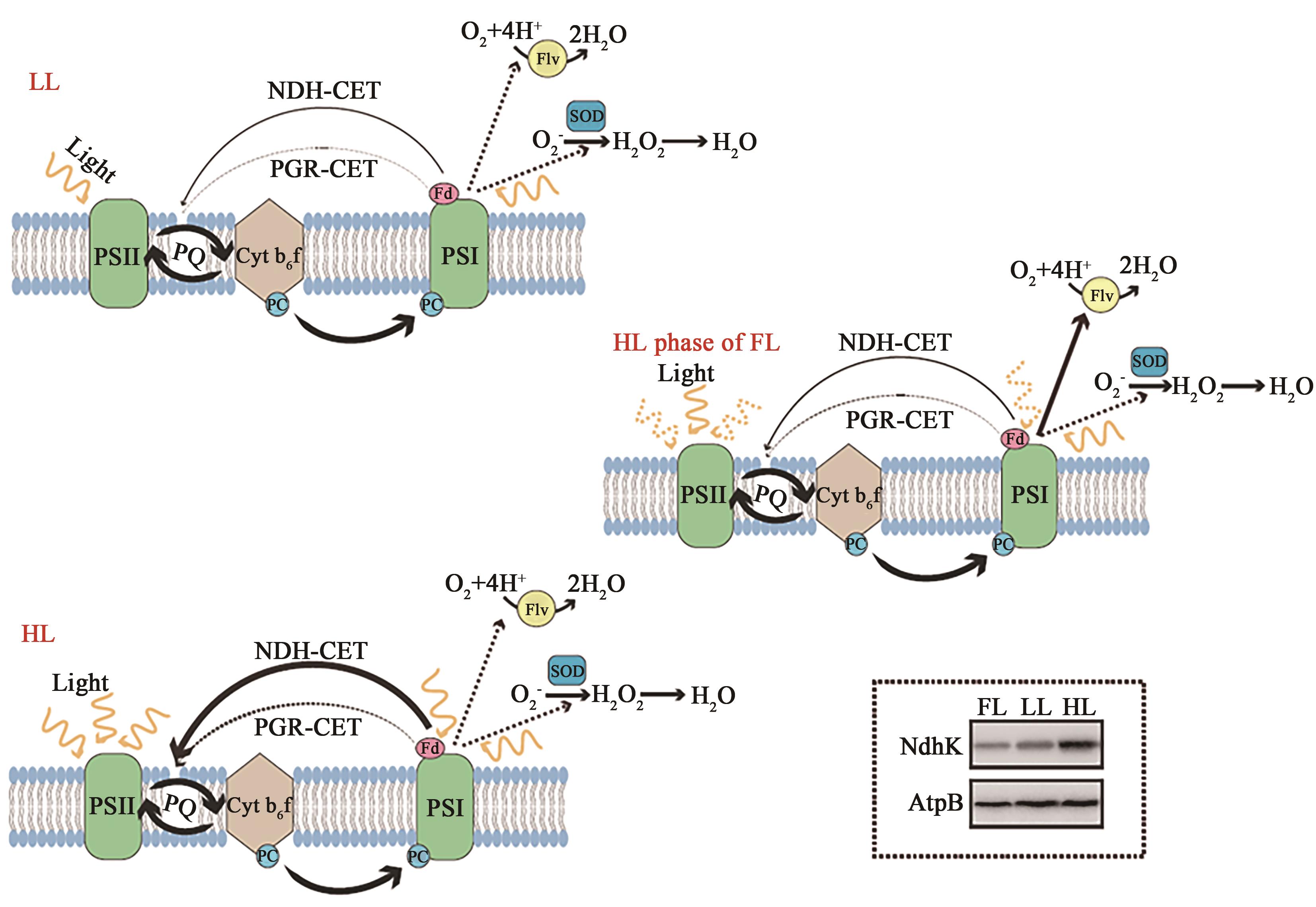
Fig. 10 Working scheme of CET, Mehler-like reaction and SOD in cyanobacteriaNote: Arrows represents electron flows and thickness represents amount of electron flows. Accumulation of NdhK representing NDH-1 complexes of WT grown under LL (20 μmol photons·m-2·s-1), HL (300 μmol photons·m-2·s-1) and FL made by Western blot was shown in right corner.
| 1 | SANE P V, IVANOV A G, ÖQUIST G, et al.. Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation [M]. Dordrecht: Springer, 2012:445-474. |
| 2 | VASS I. Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex [J]. Physiol. Plant., 2011, 142(1):6-16. |
| 3 | VASS I, STYRING S, HUNDAL T, et al.. Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promote chlorophyll triplet formation [J]. Proc. Natl. Acad. Sci. USA, 1992, 89(4):1408-1412. |
| 4 | ASADA K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons [J]. Annu. Rev. Plant Physiol. Plant Mol. Biol., 1999, 50(1):601-639. |
| 5 | ALRIC J, JOHNSON K. Alternative electron transport pathways in photosynthesis:a confluence of regulation [J]. Curr. Opin. Plant. Biol., 2017, 37:78-86. |
| 6 | MULLINEAUX C W. Electron transport and light-harvesting switches in cyanobacteria [J/OL]. Front. Plant Sci., 2014, 5∶7 [2021-04-10]. . |
| 7 | LATIFI A, RUIZ M, ZHANG C C. Oxidative stress in cyanobacteria [J]. FEMS Microbiol. Rev., 2009, 33(2):258-278. |
| 8 | PELTIER G, TOLLETER D, BILLON E, et al.. Auxiliary electron transport pathways in chloroplasts of microalgae [J]. Photosynth. Res., 2010, 106(1-2):19-31. |
| 9 | YAMORI W, SHIKANAI T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth [J]. Annu. Rev. Plant Biol., 2016, 67(1):81-106. |
| 10 | MI H, ENDO T, SCHREIBER U, et al.. Electron donation from cyclic and respiratory flows to the photosynthetic Intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium synechocystis PCC 6803 [J]. Plant Cell Physiol., 1992, 33(8):1233-1237. |
| 11 | SHIKANAI T, ENDO T, HASHIMOTO T, et al.. Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I [J]. Proc. Natl. Acad. Sci. USA, 1998, 95(16):9705-9709. |
| 12 | MUNEKAGE Y, HOJO M, MEURER J, et al.. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis [J]. Cell, 2002, 110(3):361-371. |
| 13 | YEREMENKO N, JEANJEAN R, PROMMEENATE P, et al.. Open reading frame ssr2016 is required for antimycin A-sensitive photosystem I-driven cyclic electron flow in the cyanobacterium Synechocystis sp. PCC 6803 [J]. Plant Cell Physiol., 2015, 46(8):1433-1436. |
| 14 | ARNON D I, ALLEN M B, WHATLEY F R. Photosynthesis by isolated chloroplasts [J]. Nature, 1954, 174(4426):394-396. |
| 15 | MUNEKAGE Y, HASHIMOTO M, MIYAKE C, et al.. Cyclic electron flow around photosystem I is essential for photosynthesis [J]. Nature, 2004, 429(6991):579-582. |
| 16 | OHKAWA H, PAKRASI H B, OGAWA T. Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803 [J]. J. Biol. Chem., 2000, 257(41):31630-31634. |
| 17 | ZHANG P, BATTCHIKOVA N, JANSEN T, et al.. Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803 [J]. Plant Cell, 2004, 16(12):3326-3340. |
| 18 | BATTCHIKOVA N, EISENHUT M, ARO E M. Cyanobacterial NDH-1 complexes: novel insights and remaining puzzles [J]. Biochim. Biophys. Acta, 2011, 1807(8):935-944. |
| 19 | BERNÁT G, APPEL J, OGAWA T, et al.. Distinct roles of multiple NDH-1 complexes in the cyanobacterial electron transport network as revealed by kinetic analysis of P700+ reduction in various Ndh-deficient mutants of Synechocystis sp. strain PCC6803 [J]. J. Bacteriol., 2011, 193(1):292-295. |
| 20 | PELTIER G, ARO E M, SHIKANAI T. NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis [J]. Annu. Rev. Plant Biol., 2016, 67(4):55-80. |
| 21 | MEHLER A H. Studies on reactions of illuminated chloroplasts: I. mechanism of the reduction of oxygen and other hill reagents [J]. Arch. Biochem. Biophys., 1951, 33(1):65-77. |
| 22 | ALLAHVERDIYEVA Y, SUORSA M, TIKKANEN M, et al.. Photoprotection of photosystems in fluctuating light intensities [J]. J. Exp. Bot., 2015, 66(9):2427-2436. |
| 23 | ALLAHVERDIYEVA Y, ERMAKOVA M, EISENHUT M, et al.. Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803 [J]. J. Biol. Chem., 2011, 286(27):24007-24014. |
| 24 | HELMAN Y, TCHERNOV D, REINHOLD L, et al.. Genes encoding A-type flavoproteins are essential for photoreduction of O 2 - in cyanobacteria [J]. Curr. Biol., 2013, 13(3):230-235. |
| 25 | ALLAHVERDIYEVA Y, MUSTILA H, ERMAKOVA M, et al.. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light [J]. Proc. Natl. Acad. Sci. USA, 2013,110(10):4111-4116. |
| 26 | CHA M K, HONG S K, KIM I H. Four thiol peroxidases contain a conserved GCT catalytic motif and act as a versatile array of lipid peroxidases in Anabaena sp. PCC7120 [J]. Free Radic. Biol. Med., 2007, 42(11):1736-1748. |
| 27 | PRIYA B, PREMANANDH J, DHANALAKSHMI R T, et al.. Comparative analysis of cyanobacterial superoxide dismutases to discriminate canonical forms [J/OL]. BMC Genomics, 2007, 8:435 [2021-04-10]. . |
| 28 | TICHY M, VERMAAS W. In vivo role of catalase-peroxidase in Synechocystis sp. strain PCC 6803 [J]. J. Bacteriol., 1999, 181(6):1875-1882. |
| 29 | KE W T, DAI G Z, JIANG H B, et al.. Essential roles of iron superoxide dismutase in photoautotrophic growth of Synechocystis sp. PCC 6803 and heterogeneous expression of marine Synechococcus sp. CC9311 copper/zinc superoxide dismutase within its sodB knockdown mutant [J]. Microbiology, 2014, 160(Pt 1):228-241. |
| 30 | WILLIAMS J G W. Construction of Specific Mutations in Photosystem II Photosynthetic Reaction Center by Genetic Engineering Methods in Synechocystis 6803 [M]. Cyanobacteria: Academic Press, 1988:766-778. |
| 31 | OGAWA T. A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon transport of Synechocystis PCC6803 [J]. Proc. Natl. Acad. Sci. USA, 1991, 88(10):4275-4279. |
| 32 | EISENHUT M, GEORG J, KLÄHN S, et al.. The antisense RNA As1_flv4 in the Cyanobacterium synechocystis sp. PCC 6803 prevents premature expression of the flv4-2 operon upon shift in inorganic carbon supply [J]. J. Biol. Chem., 2012, 287(40):33153-33162. |
| 33 | KAMEI A, YUASA T, ORIKAWA K, et al.. A eukaryotic-type protein kinase, SpkA, is required for normal motility of the unicellular Cyanobacterium synechocystis sp. strain PCC 6803 [J]. J. Bacteriol., 2001, 183(5):1505-1510. |
| 34 | ZHANG P, SICORA C I, VORONTSOVA N, et al.. FtsH protease is required for induction of inorganic carbon acquisition complexes in Synechocystis sp. PCC 6803 [J]. Mol. Microbiol., 2007, 65(3):728-740. |
| 35 | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method [J]. Methods, 2001, 25(4):402-408. |
| 36 | ZHANG P, ALLAHVERDIYEVA Y, EISENHUT M, et al.. Flavodiiron proteins in oxygenic photosynthetic organisms:photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803 [J/OL]. PloS One, 2009, 4(4):e5331 [2021-04-10]. . |
| 37 | MARKLUND S, MARKLUND G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase [J]. Eur. J. Biochem., 1974, 47(3):469-474. |
| 38 | LAEMMLI U K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4 [J]. Nature, 1970, 227(5259):680-685. |
| 39 | GROSSMAN A R, SCHAEFER M R, CHIANG G G, et al.. The phycobilisome, a light-harvesting complex responsive to environmental conditions [J]. Microbiol Rev., 1993, 57(3):725-749. |
| 40 | THOMAS J C, UGHY B, LAGOUTTE B, et al.. A second isoform of the ferredoxin: NADP oxidoreductase generated by an in-frame initiation of translation [J]. Proc. Natl. Acad. Sci. USA, 2006, 103(48):18368-18373. |
| 41 | EISENHUT M, AGUIRRE VON WOBESER E, JONAS L, et al.. Long-term response toward inorganic carbon limitation in wild type and glycolate turnover mutants of the Cyanobacterium synechocystis sp. strain PCC 6803 [J]. Plant Physiol., 2007, 144(4):1946-1959. |
| 42 | MI H, ENDO T, OGAWA T, et al.. Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the Cyanobacterium synechocystis sp. PCC 6803 [J]. Plant Cell Physiol., 1995, 36(4):661-668. |
| 43 | DALCORSO G, PESARESI P, MASIERO S, et al.. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis [J]. Cell, 2008, 132(2):273-285. |
| 44 | GAO F, ZHAO J, CHEN L, et al.. The NDH-1L-PSI supercomplex is important for efficient cyclic electron transport in cyanobacteria [J]. Plant Physiol., 2016,172(3):1451-1464. |
| 45 | COOLEY J W, HOWITT C A, VERMAAS W F J. Succinate:quinol oxidoreductases in the Cyanobacterium synechocystis sp. strain PCC 6803: presence and function in metabolism and electron transport [J]. J. Bacteriol., 2000, 182(3):714-722. |
| 46 | HERRANEN M, BATTCHIKOVA N, ZHANG P, et al.. Towards functional proteomics of membrane protein complexes in Synechocystis sp. PCC 6803 [J]. Plant Physiol., 2004, 134(1):470-481. |
| 47 | ZHAO J, GAO F, ZHANG J, et al.. NdhO, A subunit of NADPH dehydrogenase, destabilizes medium size complex of the enzyme in Synechocystis sp. strain PCC 6803 [J]. J. Biol. Chem., 2014:289(39):26669-26676. |
| 48 | NISHIYAMA Y, ALLAKHVERDIEV S I, YAMAMOTO H, et al.. Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803 [J]. Biochemistry, 2004, 43(35):11321-11330. |
| 49 | REGELSBERGER G, JAKOPITSCH C, PLASSER L, et al.. Occurrence and biochemistry of hydroperoxidases in oxygenic phototrophic prokaryotes (cyanobacteria) [J]. Plant Physiol. Biochem., 2002, 40(6):479-490. |
| 50 | RYOSUKE H, GINGA S, KEIICHIRO S, et al.. O2-dependent large electron flow functioned as an electron sink, replacing the steady-state electron flux in photosynthesis in the Cyanobacterium synechocystis sp. PCC 6803, but not in the Cyanobacterium synechococcus sp. PCC 7942 [J]. Biosci. Biotechnol. Biochem., 2014, 78(3):384-393. |
| 51 | DAUTERMANN O, LOHR M. A functional zeaxanthin epoxidase from red algae shedding light on the evolution of light-harvesting carotenoids and the xanthophyll cycle in photosynthetic eukaryotes [J]. Plant J., 2017, 92(5):879-891. |
| 52 | STORTI M, ALBORESI A, GEROTTO C, et al.. Role of cyclic and pseudo-cyclic electron transport in response to dynamic light changes in Physcomitrella patens [J]. Plant Cell Environ., 2019, 42(5):1590-1602. |
| 53 | YAMAMOTO H, SHIKANAI T. PGR5-Dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides [J]. Plant Physiol., 2019, 179(2):588-600. |
| [1] | YANG Ting1,2, REN Chun-ying3, LI Juan4, YAN Yan-chun2, ZHAO Hong-xin3, WU Kun1*,. Isolation and Screening of Oleaginous Microalgae from Municipal Wastewater [J]. , 2015, 17(3): 144-151. |
| [2] | SUN Zhi\|lan1, HUANG Yun1,2, CHEN Yi\|feng1*. Progress of Air Fertilizer Photobioreactor of Microalgae [J]. , 2014, 16(6): 117-123. |
| [3] | SUN Chuan-fan . Research Progress on Microalgae Rehabilitation of Water Environment [J]. , 2011, 13(3): 92-96. |
| [4] | LI Hai-feng1, LI Zhang-wei1,2, LIU Yong-mei1, HUANG Ze-bo1. Prospect for Present Status and Application of Polysaccharides from Nostoc [J]. , 2011, 13(1): 105-110. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号