




















Journal of Agricultural Science and Technology ›› 2023, Vol. 25 ›› Issue (10): 152-164.DOI: 10.13304/j.nykjdb.2022.0121
• ANIMAL AND PLANT HEALTH • Previous Articles
Hangfan GUO1( ), Ping WANG1,2(
), Ping WANG1,2( ), Ying WANG3
), Ying WANG3
Received:2022-02-23
Accepted:2022-05-07
Online:2023-10-15
Published:2023-10-27
Contact:
Ping WANG
通讯作者:
王萍
作者简介:郭航帆 E-mail 1049077469@qq.com;
CLC Number:
Hangfan GUO, Ping WANG, Ying WANG. Inhibitory Effect of Solanum nigrum L. Extracts on Escherichia coli and Enterococcus faecalis and Biofilm Formation[J]. Journal of Agricultural Science and Technology, 2023, 25(10): 152-164.
郭航帆, 王萍, 王颖. 龙葵提取物对大肠杆菌、粪肠球菌及其生物膜形成的抑制作用[J]. 中国农业科技导报, 2023, 25(10): 152-164.
| 因素Factor | 水平 Level | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A:提取温度Temperature/℃ | 20 | 30 | 40 |
| B:乙醇含量Ethanol content/% | 40 | 45 | 50 |
| C:液料比Liquid-solid ratio/(mL·g-1) | 4∶1 | 5∶1 | 6∶1 |
Table 1 Factors and levels in the experimental design
| 因素Factor | 水平 Level | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A:提取温度Temperature/℃ | 20 | 30 | 40 |
| B:乙醇含量Ethanol content/% | 40 | 45 | 50 |
| C:液料比Liquid-solid ratio/(mL·g-1) | 4∶1 | 5∶1 | 6∶1 |
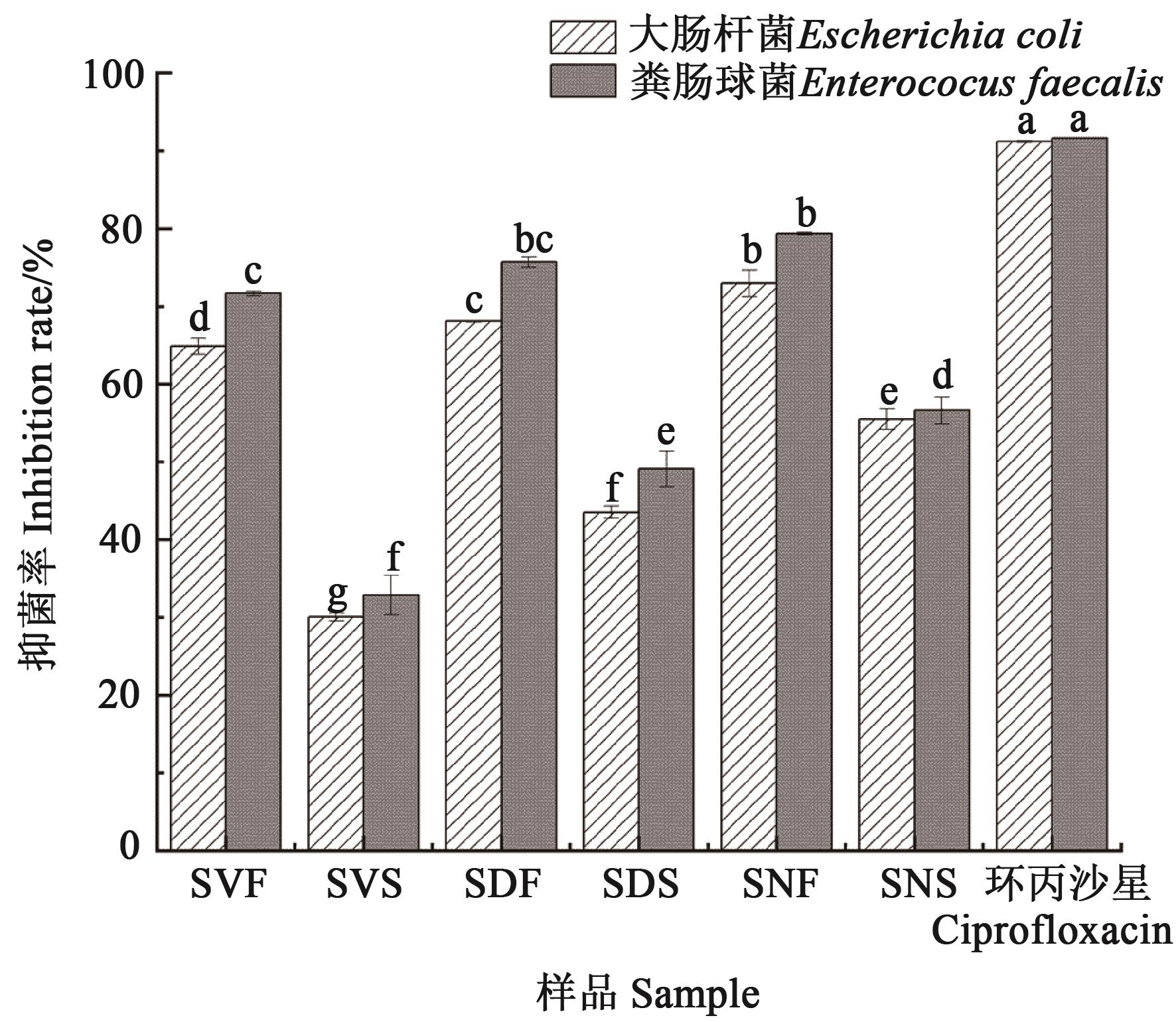
Fig. 1 Inhibition of extracts from different parts of Solanum nigrum L. on the growth of Escherichia coli and Enterococcus faecalisNote:Different lowercase letters indicate significant differences between different treatments of same bacteria at P<0.05 level.
| 样品Sample | 单宁Tannin | 皂苷Saponin | 蒽醌Anthraquinone | 甾醇 Sterol | 黄酮Flavone | 萜类Terpenoids | 生物碱Alkaloid | |
|---|---|---|---|---|---|---|---|---|
Wanger’s检测 Wanger’s test | Dragendorff’s检测 Dragendorff’s test | |||||||
| SNF | + | ++ | - | + | + | ++ | + | + |
| SNS | + | + | - | + | + | + | + | + |
| SDF | + | ++ | - | + | + | ++ | + | + |
| SDS | + | + | - | + | + | + | + | + |
| SVF | + | ++ | - | + | + | ++ | + | + |
| SVS | + | + | - | + | + | + | + | + |
Table 2 Phytochemical screening of Solanum nigrum L. extract
| 样品Sample | 单宁Tannin | 皂苷Saponin | 蒽醌Anthraquinone | 甾醇 Sterol | 黄酮Flavone | 萜类Terpenoids | 生物碱Alkaloid | |
|---|---|---|---|---|---|---|---|---|
Wanger’s检测 Wanger’s test | Dragendorff’s检测 Dragendorff’s test | |||||||
| SNF | + | ++ | - | + | + | ++ | + | + |
| SNS | + | + | - | + | + | + | + | + |
| SDF | + | ++ | - | + | + | ++ | + | + |
| SDS | + | + | - | + | + | + | + | + |
| SVF | + | ++ | - | + | + | ++ | + | + |
| SVS | + | + | - | + | + | + | + | + |
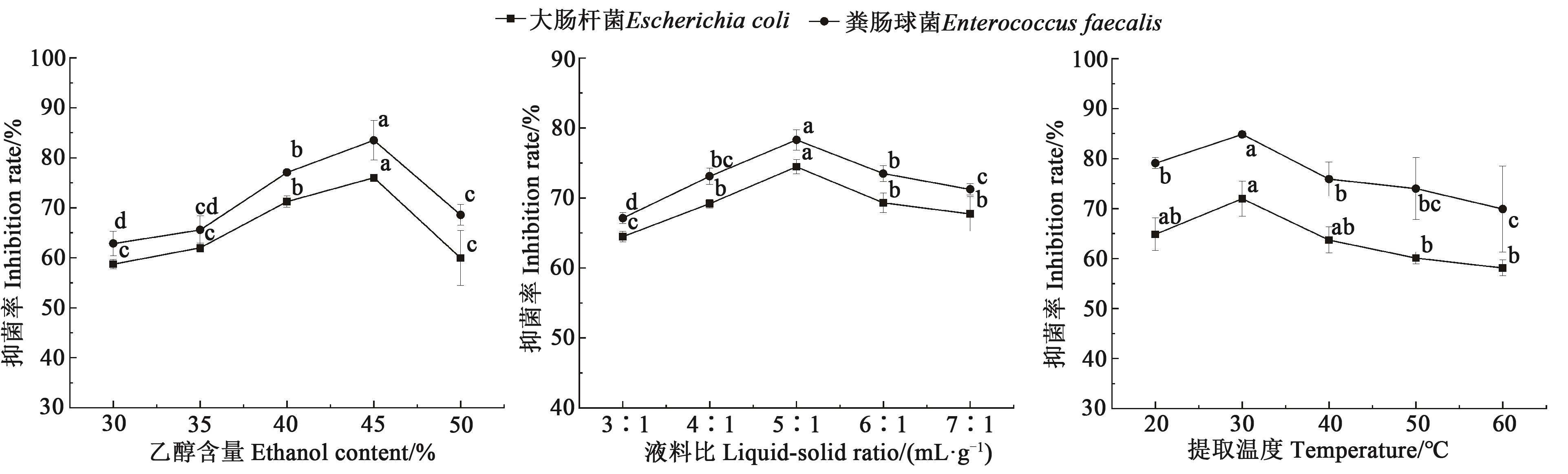
Fig. 2 Results of single factor experimentsNote:Different lowercase letters indicate significant differences between different treatments of same bacteria at P<0.05 level.
| 试验编号 No. | A:提取温度Temperature | B:乙醇含量Ethanol content | C:液料比 Liquid-solid ratio | 抑制率Inhibition rate/% | |
|---|---|---|---|---|---|
Y1:大肠杆菌 Escherichia coli | Y2:粪肠球菌 Enterococcus faecalis | ||||
| 1 | 1 | 0 | 1 | 76.92±0.58 | 83.54±0.82 |
| 2 | 0 | 0 | 0 | 80.64±0.53 | 88.85±0.76 |
| 3 | 1 | -1 | 0 | 75.16±0.24 | 82.05±0.57 |
| 4 | -1 | -1 | 0 | 75.86±0.60 | 82.32±0.46 |
| 5 | 0 | 1 | -1 | 72.10±0.49 | 78.95±0.58 |
| 6 | -1 | 0 | 1 | 77.66±0.72 | 74.22±0.77 |
| 7 | 0 | 1 | 1 | 72.73±0.31 | 79.53±0.31 |
| 8 | 0 | 0 | 0 | 78.76±0.57 | 88.75±0.39 |
| 9 | 0 | -1 | 1 | 74.69±0.45 | 81.60±0.88 |
| 10 | -1 | 1 | 0 | 74.06±0.78 | 81.20±0.93 |
| 11 | 1 | 0 | -1 | 75.71±1.00 | 82.39±0.53 |
| 12 | 0 | 0 | 0 | 79.98±0.85 | 88.71±0.39 |
| 13 | -1 | 0 | -1 | 76.14±0.47 | 82.83±0.82 |
| 14 | 0 | 0 | 0 | 78.84±0.77 | 89.36±0.31 |
| 15 | 1 | 1 | 0 | 73.63±0.60 | 81.16±0.42 |
| 16 | 0 | -1 | -1 | 73.75±0.48 | 80.65±0.72 |
| 17 | 0 | 0 | 0 | 79.55±0.79 | 89.09±0.57 |
Table 3 Programs and experiment results of the Box-Behnken design
| 试验编号 No. | A:提取温度Temperature | B:乙醇含量Ethanol content | C:液料比 Liquid-solid ratio | 抑制率Inhibition rate/% | |
|---|---|---|---|---|---|
Y1:大肠杆菌 Escherichia coli | Y2:粪肠球菌 Enterococcus faecalis | ||||
| 1 | 1 | 0 | 1 | 76.92±0.58 | 83.54±0.82 |
| 2 | 0 | 0 | 0 | 80.64±0.53 | 88.85±0.76 |
| 3 | 1 | -1 | 0 | 75.16±0.24 | 82.05±0.57 |
| 4 | -1 | -1 | 0 | 75.86±0.60 | 82.32±0.46 |
| 5 | 0 | 1 | -1 | 72.10±0.49 | 78.95±0.58 |
| 6 | -1 | 0 | 1 | 77.66±0.72 | 74.22±0.77 |
| 7 | 0 | 1 | 1 | 72.73±0.31 | 79.53±0.31 |
| 8 | 0 | 0 | 0 | 78.76±0.57 | 88.75±0.39 |
| 9 | 0 | -1 | 1 | 74.69±0.45 | 81.60±0.88 |
| 10 | -1 | 1 | 0 | 74.06±0.78 | 81.20±0.93 |
| 11 | 1 | 0 | -1 | 75.71±1.00 | 82.39±0.53 |
| 12 | 0 | 0 | 0 | 79.98±0.85 | 88.71±0.39 |
| 13 | -1 | 0 | -1 | 76.14±0.47 | 82.83±0.82 |
| 14 | 0 | 0 | 0 | 78.84±0.77 | 89.36±0.31 |
| 15 | 1 | 1 | 0 | 73.63±0.60 | 81.16±0.42 |
| 16 | 0 | -1 | -1 | 73.75±0.48 | 80.65±0.72 |
| 17 | 0 | 0 | 0 | 79.55±0.79 | 89.09±0.57 |
方差来源 Variance source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方 Mean square | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| 模型Model | 108.440 | 9 | 12.050 | 31.480 | <0.000 1 | ** |
| A | 0.660 | 1 | 0.660 | 1.730 | 0.230 1 | NS |
| B | 6.020 | 1 | 6.020 | 15.730 | 0.005 4 | ** |
| C | 2.310 | 1 | 2.310 | 6.040 | 0.043 6 | * |
| AB | 0.018 | 1 | 0.018 | 0.048 | 0.833 5 | NS |
| AC | 0.024 | 1 | 0.024 | 0.063 | 0.809 4 | NS |
| BC | 0.024 | 1 | 0.024 | 0.063 | 0.809 4 | NS |
| A2 | 2.650 | 1 | 2.650 | 6.920 | 0.033 9 | * |
| B2 | 70.200 | 1 | 70.200 | 183.410 | <0.000 1 | ** |
| C2 | 19.520 | 1 | 19.520 | 51.000 | 0.000 2 | ** |
| 残差Residual | 2.680 | 7 | 0.380 | |||
| 失拟项Lack of fit | 0.180 | 3 | 0.059 | 0.095 | 0.958 8 | NS |
| 纯误差Pure error | 2.500 | 4 | 0.630 | |||
| 总和Total | 111.120 | 16 |
Table 4 ANOVA of the regression equation model of Escherichia coli inhibition rate
方差来源 Variance source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方 Mean square | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| 模型Model | 108.440 | 9 | 12.050 | 31.480 | <0.000 1 | ** |
| A | 0.660 | 1 | 0.660 | 1.730 | 0.230 1 | NS |
| B | 6.020 | 1 | 6.020 | 15.730 | 0.005 4 | ** |
| C | 2.310 | 1 | 2.310 | 6.040 | 0.043 6 | * |
| AB | 0.018 | 1 | 0.018 | 0.048 | 0.833 5 | NS |
| AC | 0.024 | 1 | 0.024 | 0.063 | 0.809 4 | NS |
| BC | 0.024 | 1 | 0.024 | 0.063 | 0.809 4 | NS |
| A2 | 2.650 | 1 | 2.650 | 6.920 | 0.033 9 | * |
| B2 | 70.200 | 1 | 70.200 | 183.410 | <0.000 1 | ** |
| C2 | 19.520 | 1 | 19.520 | 51.000 | 0.000 2 | ** |
| 残差Residual | 2.680 | 7 | 0.380 | |||
| 失拟项Lack of fit | 0.180 | 3 | 0.059 | 0.095 | 0.958 8 | NS |
| 纯误差Pure error | 2.500 | 4 | 0.630 | |||
| 总和Total | 111.120 | 16 |
方差来源 Variance source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方 Mean square | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| 模型Model | 209.990 | 9 | 23.330 | 197.910 | <0.000 1 | ** |
| A | 0.260 | 1 | 0.260 | 2.170 | 0.184 4 | NS |
| B | 4.030 | 1 | 4.030 | 34.210 | 0.000 6 | ** |
| C | 2.170 | 1 | 2.170 | 18.440 | 0.003 6 | ** |
| AB | 0.010 | 1 | 0.010 | 0.110 | 0.747 5 | NS |
| AC | 0.010 | 1 | 0.010 | 0.120 | 0.737 0 | NS |
| BC | 0.020 | 1 | 0.020 | 0.150 | 0.705 9 | NS |
| A2 | 18.850 | 1 | 18.850 | 159.910 | <0.000 1 | ** |
| B2 | 111.830 | 1 | 111.830 | 948.530 | <0.000 1 | ** |
| C2 | 54.300 | 1 | 54.300 | 460.550 | <0.000 1 | ** |
| 残差Residual | 0.830 | 7 | 0.120 | |||
| 失拟项Lack of fit | 0.530 | 3 | 0.180 | 2.390 | 0.209 2 | NS |
| 纯误差Pure error | 0.300 | 4 | 0.070 | |||
| 总和Total | 210.810 | 16 |
Table 5 ANOVA of the regression equation model of Enterococcus faecalis inhibition rate
方差来源 Variance source | 平方和 Sum of squares | 自由度 Degree of freedom | 均方 Mean square | F值 F value | P值 P value | 显著性 Significance |
|---|---|---|---|---|---|---|
| 模型Model | 209.990 | 9 | 23.330 | 197.910 | <0.000 1 | ** |
| A | 0.260 | 1 | 0.260 | 2.170 | 0.184 4 | NS |
| B | 4.030 | 1 | 4.030 | 34.210 | 0.000 6 | ** |
| C | 2.170 | 1 | 2.170 | 18.440 | 0.003 6 | ** |
| AB | 0.010 | 1 | 0.010 | 0.110 | 0.747 5 | NS |
| AC | 0.010 | 1 | 0.010 | 0.120 | 0.737 0 | NS |
| BC | 0.020 | 1 | 0.020 | 0.150 | 0.705 9 | NS |
| A2 | 18.850 | 1 | 18.850 | 159.910 | <0.000 1 | ** |
| B2 | 111.830 | 1 | 111.830 | 948.530 | <0.000 1 | ** |
| C2 | 54.300 | 1 | 54.300 | 460.550 | <0.000 1 | ** |
| 残差Residual | 0.830 | 7 | 0.120 | |||
| 失拟项Lack of fit | 0.530 | 3 | 0.180 | 2.390 | 0.209 2 | NS |
| 纯误差Pure error | 0.300 | 4 | 0.070 | |||
| 总和Total | 210.810 | 16 |
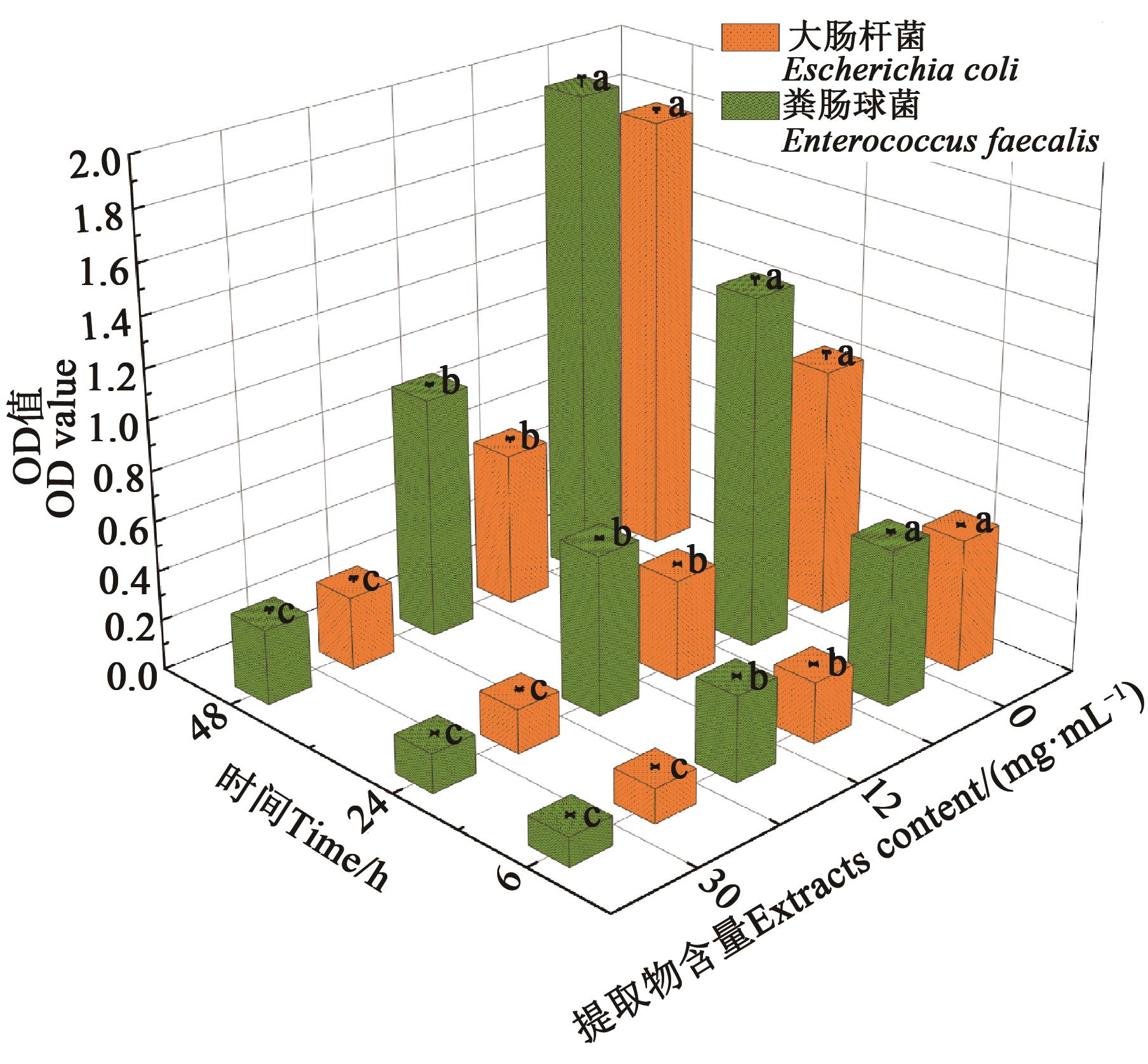
Fig. 5 Effect of Solanum nigrum L. fruit extracts on bacterial biofilm formationNote:Different lowercase letters indicate significant differences between different treatments of same bacteria at same time at P<0.05 level.
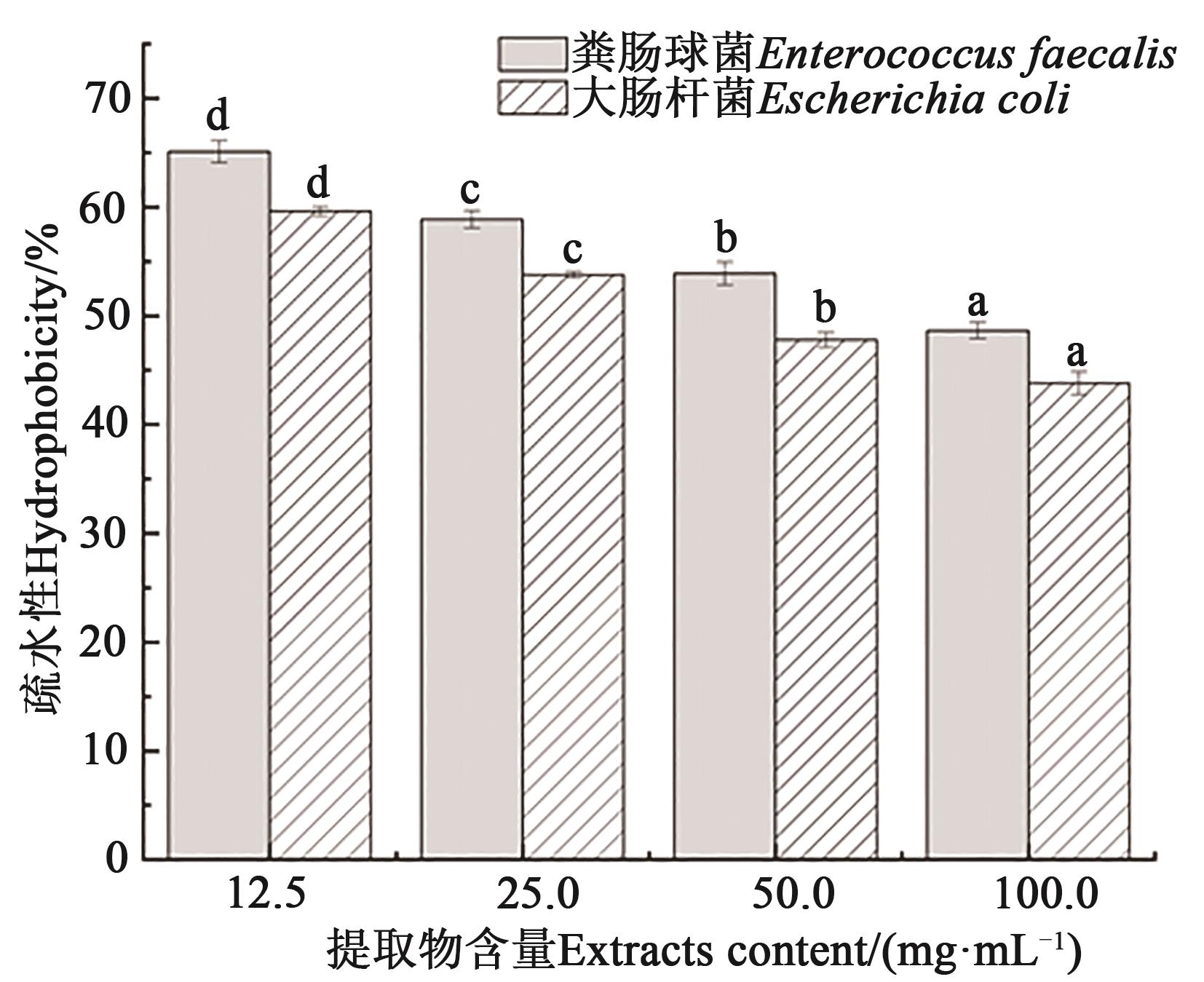
Fig. 6 Effect of Solanum nigrum L. fruit extracts on hydrophobicityNote:Different lowercase letters indicate significant differences between different treatments of same bacteria at P < 0.05 level.
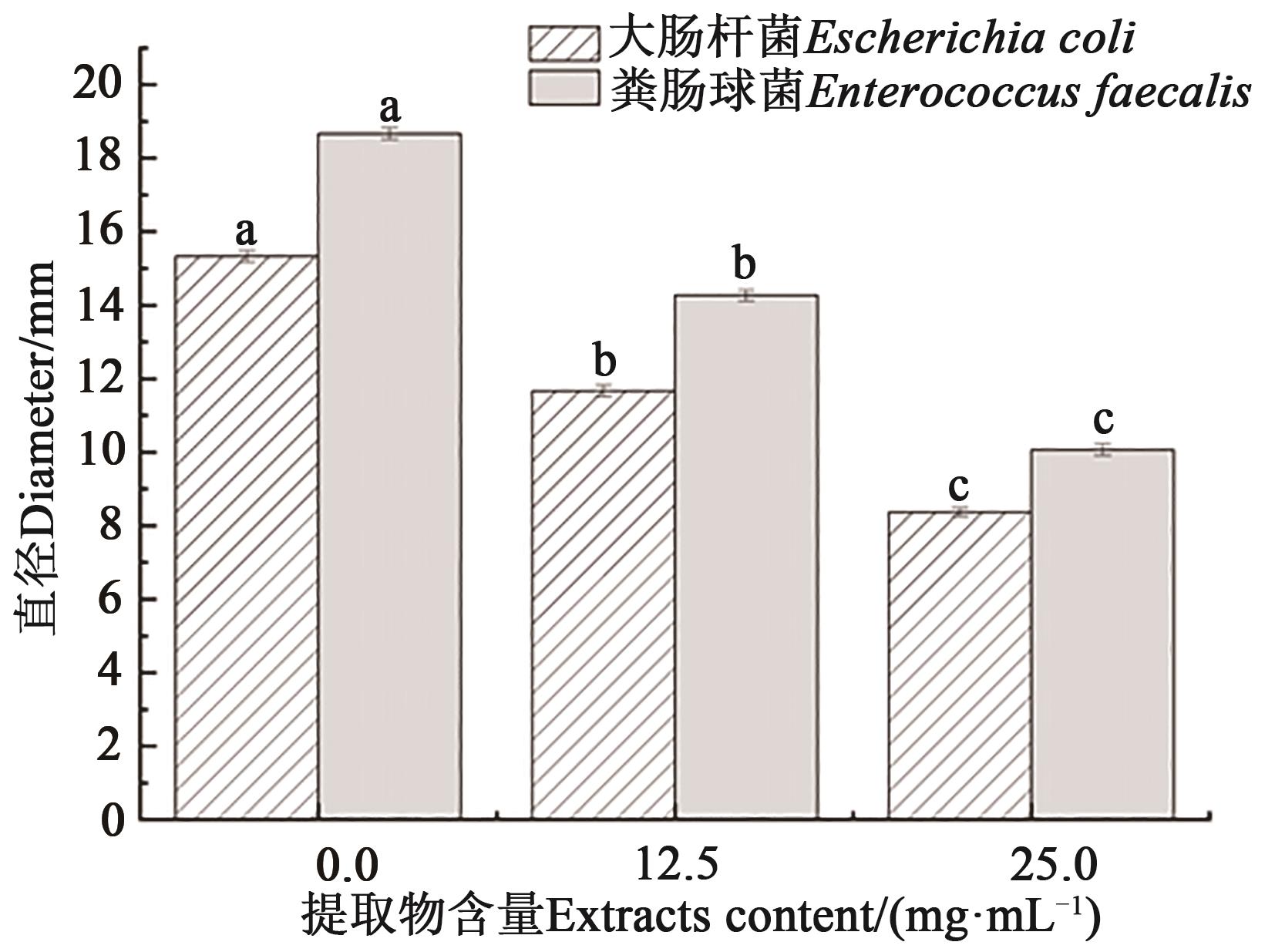
Fig. 7 Effect of Solanum nigrum L. fruit extracts on swimmingNote:Different lowercase letters indicate significant differences between different treatments of same bacteria at P<0.05 level.
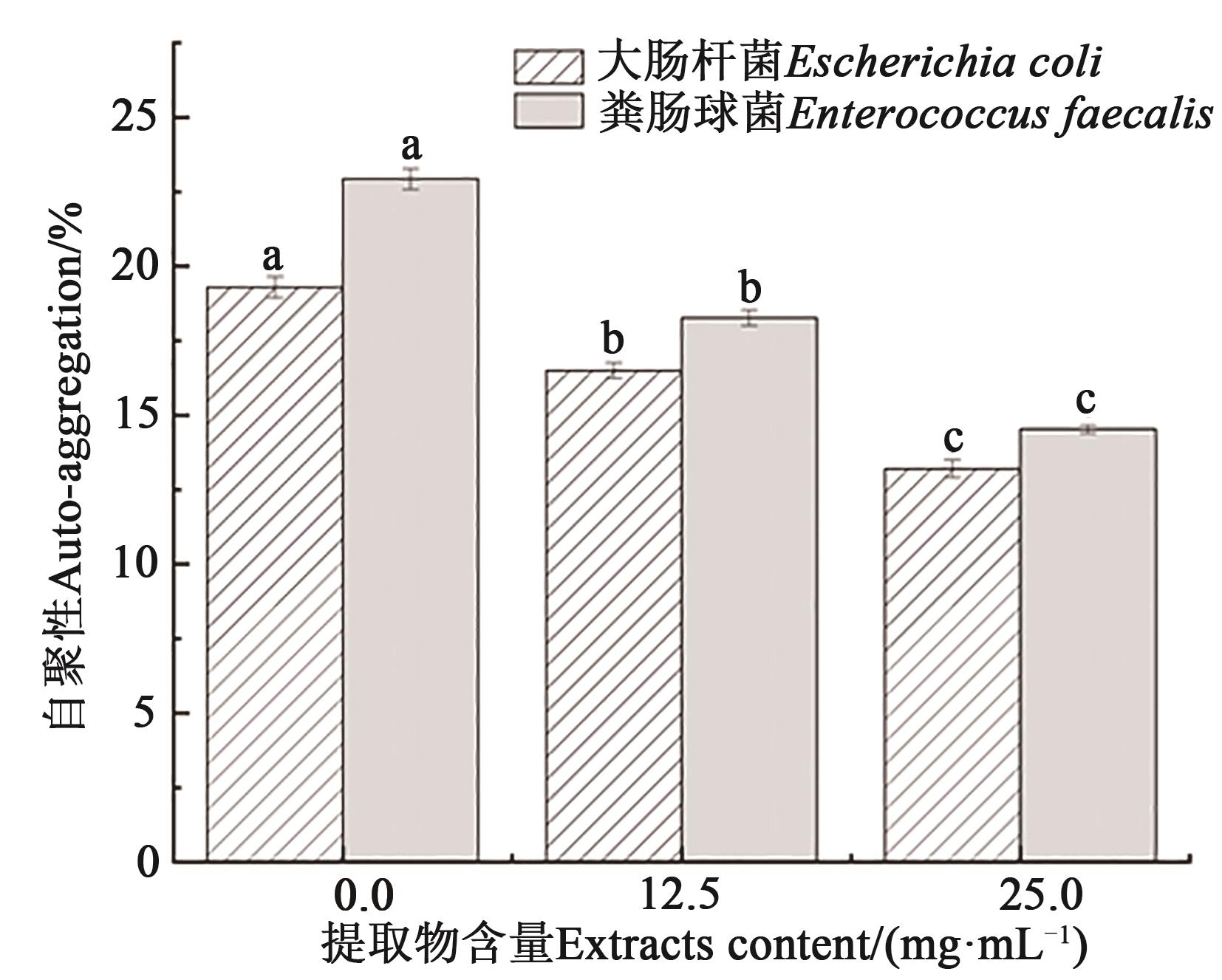
Fig. 8 Effect of Solanum nigrum L. fruit extracts on auto-aggregationNote:Different lowercase letters indicate significant differences between different treatments of same bacteria at P<0.05 level.
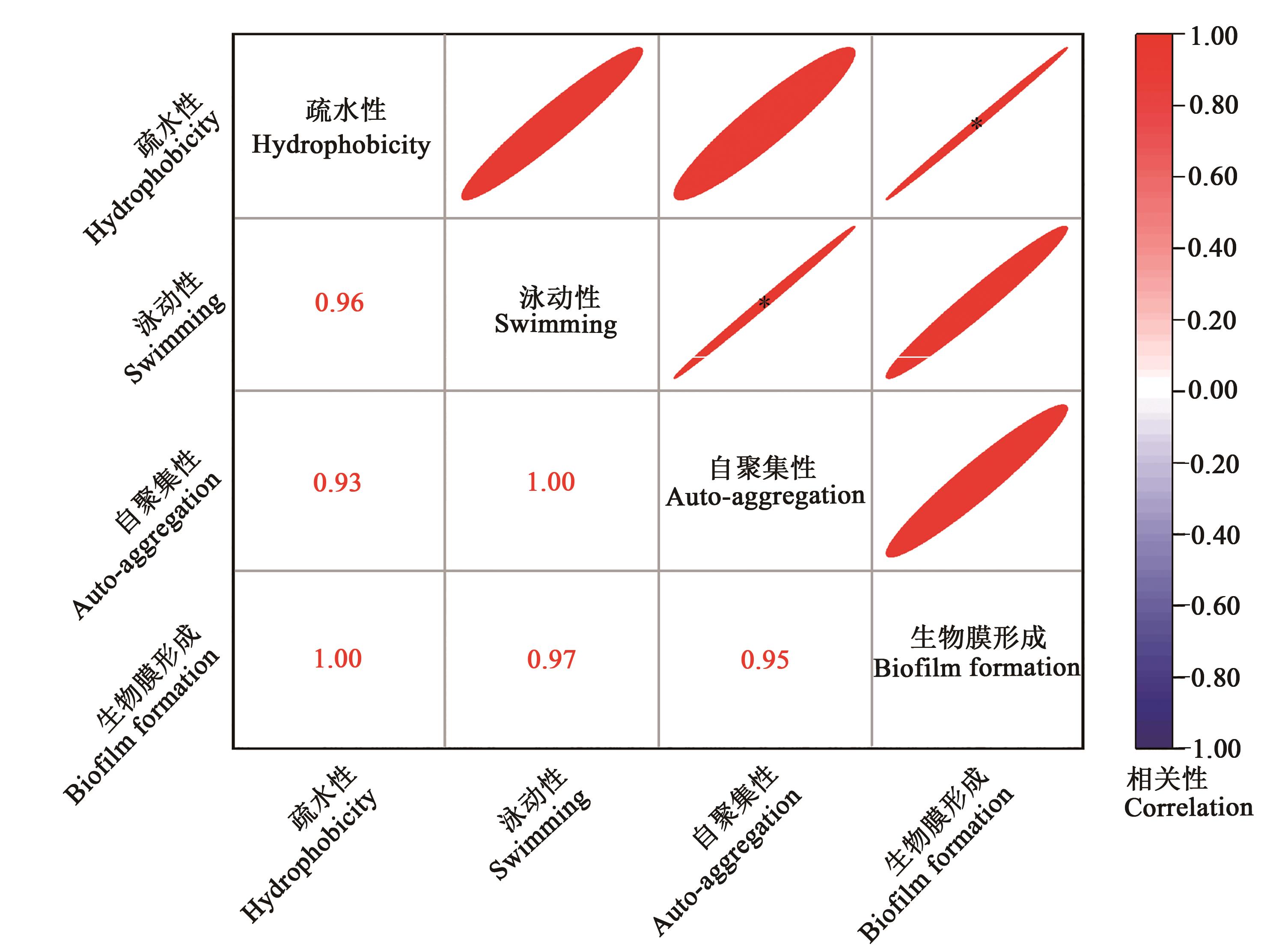
Fig. 9 Correlation analysis between biofilm formation ability and physicochemical properties of Escherichia coliNote:* indicates significant correlation at P<0.05 level.

Fig. 10 Correlation analysis between biofilm formation ability and physicochemical properties of Enterococcus faecalisNote: * indicates significant correlation at P< 0.05 level.
| 1 | 武喜红,李雪.《本草纲目》中茄科药物基源考证[J].亚太传统医药, 2018,14(11):85-86. |
| WU X H, LI X. Research on the medicinal base source of Solanaceae in Compendium of Materia Medica [J]. Asia-Pacific Traditional Med., 2018,14(11): 85-86. | |
| 2 | JABAMALAIRAJ A, PRIATAMA R A, HEO J, et al.. Medicinal metabolites with common biosynthetic pathways in Solanum nigrum [J]. Plant Biotechnol. Rep., 2019,13(4):315-327. |
| 3 | ILONDU E M, BOSAH B O. Fungicidal activity of Solanum nigrum and Physalis angulata extracts against Macrophomina phaseolina, a fruit rot pathogen of melon (Citrullus colocynthis (L.) Schrad [J]. J. Biopesticides, 2017,10(2):135-139. |
| 4 | CHESTER K, ZAHIREDDIN S, AHMAD A, et al.. Bioautography-based identification of antioxidant metabolites of Solanum nigrum L. and exploration its hepatoprotective potential against D-galactosamine-induced hepatic fibrosis in rats [J]. Pharmacogn Mag., 2019,15S(62):104-110. |
| 5 | ZAGHLOOL S S, ABO-SEIF A A, RABEH M A, et al.. Gastro-protective and anti-Oxidant potential of Althaea officinalis and Solanum nigrum on pyloric ligation/indomethacin-induced ulceration in rats [J/OL]. Antioxidants, 2019,8(11):512 [2022-01-22]. . |
| 6 | CHURIYAH C, NINGSIH S, FIRDAYANI F. The cytotoxic, apoptotic Induction, and cell cycle arrest activities of Solanum nigrum L. ethanolic extract on MCF-7 human breast cancer cell [J]. Asian Pac. J. Cancer Prev., 2020,21(12):3735-3741. |
| 7 | SHIN K, EUM Y. The antioxidant and antimicrobial activity of Solanum nigrum L. fruit powder by extraction solvent [J]. Korean J. Food Nutr., 2021,34(2):137-145. |
| 8 | YEOM Y, KIM M A, KIM J, et al.. Anti-inflammatory effects of the extract of Solanum nigrum L. on an acute ear edema mouse model [J]. Materials Technol., 2019,34(14):851-857. |
| 9 | KNAPP S, BARBOZA G E, SARKINEN T. Proposals to reject the name Solanum rubrum and to conserve the name S. alatum with a conserved type (Solanaceae) [J]. Taxon, 2017,66(4):988-989. |
| 10 | ABDEL-HAMID A E E, DINE R SEI, SENDKER J, et al.. Metabolic profiling of Solanum villosum Mill subsp. miniatum (Bernh. ex Willd.): hepatoprotective and antifibrotic activity in a rat model of liver fibrosis [J]. Pharmacogn. Mag., 2019,15(65):659-670. |
| 11 | HOSSAIN S J, EL-SAYED M A, MOHAMED A H, et al.. Phenolic content, anti-oxidative, anti-alpha-amylase and anti-alpha-glucosidase activities of Solanum diphylluml [J]. Bangladesh J. Bot., 2009,38(2):139-143. |
| 12 | THIEME L, HARTUNG A, TRAMM K, et al.. MBEC versus MBIC: the lack of differentiation between biofilm reducing and inhibitory effects as a current problem in biofilm methodology [J/OL]. Biol. Proced. Online, 2019,21(1):18 [2022-01-22]. . |
| 13 | DI MARCO N I, PAEZ P L, LUCERO-ESTRADA C S M, et al.. Naphthoquinones inhibit formation and viability of Yersinia enterocolitica biofilm [J/OL]. World J. Microbiol. Biotechnol., 2021,37(2):30 [2022-01-22]. . |
| 14 | CIOFU O, TOLKER-NIELSEN T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics [J/OL]. Front. Microbiol., 2019,10:913 [2022-01-22]. . |
| 15 | MIZAN M F R, JAHID I K, KIM M, et al.. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation [J]. Biofouling, 2016,32(4):497-509. |
| 16 | CALLE A F. Effect of anthocyanins in okinawan sweet potato on gowth and physicochemical properties of Salmonella typhimurium and Listeria monocytogenes [D]. Hawaii: University of Hawaii, 2020. |
| 17 | KHALEGHI M, KHORRAMI S. Down-regulation of biofilm-associated genes in mecA-positive methicillin-resistant S. aureus treated withM. communis extract and its antibacterial activity [J/OL]. AMB Express, 2021,11(1):85 [2022-01-22]. . |
| 18 | 梁兆超,郭显炜,宋艳娟,等.响应面法优化双孢蘑菇多糖提取工艺及其体外抗氧化活性研究[J].中国农业科技导报,2019,21(8):161-168. |
| LIANG Z C, GUO X W, SONG Y J, et al.. Extraction process of polysaccharide in Agaricus bisporus optimized by response surface method and its antioxidant activity in vitro [J]. J. Agric. Sci. Technol., 2019,21(8):161-168. | |
| 19 | 陈志迪,王新宇,李晴雯,等.植物源抑菌剂的研究进展[J].食品安全质量检测学报,2021,12(18):7433-7439. |
| CHEN Z D, WANG X Y, LI Q W, et al.. Research progress of plant-derived antimicrobial agents [J]. J. Food Saf. Qual.,2021,12(18):7433-7439. | |
| 20 | 张弘弛, 刘瑞, 柴帅, 等.恒山黄芪种子中抗菌肽提取条件的优化和抑菌率测定[J].食品科技,2021,46(3):212-219. |
| ZHANG H C, LIU R, CHAI S, et al.. Optimization of extraction conditions of antimicrobial peptides from Astragalus seeds and determination of antibacterial rate [J]. Food Sci. Technol., 2021,46(3): 212-219. | |
| 21 | MOURA D F D, ROCHA T A, BARROS D D M, et al.. Evaluation of the antioxidant, antibacterial, and antibiofilm activity of the sesquiterpene nerolidol [J]. Arch. Microbiol., 2021,203(7): 4303-4311. |
| 22 | INDHUMATHI T, MOHANDASS S. Efficacy of ethanolic extract of Solanum incanum fruit extract for its antimicrobial activity [J]. Int. J. Curr. Microbiol. Appl. Sci., 2014,3(6):939-949. |
| 23 | 李程程.牡丹花酚类成分提取纯化及其对大肠杆菌、金黄色葡萄球生物被膜抑制活性研究[D].无锡: 江南大学, 2020. |
| LI C C. Study on the extraction and purification of phenolics from peony flowers and their anti-biofilm activities of Escherichia coli and Staphylococcus aureus [D]. Wuxi: Jiangnan University, 2020. | |
| 24 | MAGNINI R D, NITIEMA M, OUEDRAOGO G G, et al.. Toxicity and bacterial anti-motility activities of the hydroethanolic extract of Acacia senegal (L.) Willd (Fabaceae) leaves [J/OL]. BMC Complementary Med. Ther., 2021,21(1): 178 [2022-01-22]. . |
| 25 | SALAHEEN S, JAISWAL E, JOO J, et al.. Bioactive extracts from berry byproducts on the pathogenicity of Salmonella Typhimurium [J]. Int. J. Food Microbiol., 2016,237:128-135. |
| 26 | PENESYAN A, PAULSEN I T, GILLINGS M R, et al.. Secondary effects of antibiotics on microbial biofilms [J/OL]. Front. Microbiol., 2020,11:2019 [2020-10-06]. . |
| 27 | KALAMARA M, ABBOTT J C, MACPHEE C E, et al.. Biofilm hydrophobicity in environmental isolates of Bacillus subtilis [J/OL]. Microbiology, 2021,167(9):441976 [2022-01-22]. . |
| 28 | COUVIGNY B, KULAKAUSKAS S, PONS N, et al.. Identification of new factors modulating adhesion abilities of the pioneer commensal bacterium Streptococcus salivarius [J/OL]. Front. Microbiol., 2018,9:273 [2022-01-22]. . |
| 29 | 张秋香,黄银,姚沛琳,等.植物乳杆菌FB-T9抑制变异链球菌及其生物膜形成的研究[J].食品与生物技术学报,2019,38(9):17-26. |
| ZHANG Q X, HUANG Y, YAO P L, et al.. Inhibition of Streptococcus mutans and its biofilm formation by Lactobacillus plantarum FB-T9 [J]. J. Food Sci. Biotechnol., 2019,38(9): 17-26. | |
| 30 | 姜春新,王雅莹,洪小利,等.柠檬酸和乙酸对致腐假单胞菌的抗生物被膜研究[J].核农学报,2021,35(1):120-127. |
| JIANG C X, WANG Y Y, HONG X L, et al.. Antibiofilm of citric acid and acetic acid against Spoilage related Pseudomonas [J]. Nucl. Agric. Sci., 2021,35(1): 120-127. | |
| 31 | 张荣先,仇博宇,赵佳,等.青刺果不同部位水提取液的抑菌效果[J].安徽农业科学,2007, 35(2):408-409, 411. |
| ZHANG R X, QIU B Y, ZHAO J, et al.. Effects of extracts from Prinsepia utilis royle on antimicrobial activity [J]. J. Anhui Agric. Sci., 2007,35(2): 408-409, 411. | |
| 32 | 姜雪琪,张卫东.龙葵超声波乙醇提取物抑菌作用研究[J].安徽农业科学,2016,44(31):125-155. |
| JIANG X Q, ZHANG W D. Study on antibacterial effect of ultrasonic ethanol extracts of Solanum nigrum [J]. J. Anhui Agric. Sci., 2016,44(31): 125-155. | |
| 33 | 张晶,邢媛媛,徐元庆,等.植物提取物活性成分的提取工艺及抑菌活性研究进展[J].动物营养学报,2019,31(12):5461-5467. |
| ZHANG J, XING Y Y, XU Y Q, et al.. Research progress on extraction technology and bacteriostatic activity of activeigredients in plant extracts [J]. Chin. J. Anim. Nutr., 2019,31(12):5461-5467. | |
| 34 | KALAISELVI I, RAJALAKSHMI P, PUSHPA S M, et al.. Enhanced bioaccessibility of green tea polyphenols and lipophilic activity of EGCG octaacetate on gram-negative bacteria [J]. LWT Food Sci. Technol., 2019,105:103-109. |
| 35 | MAMATOVA A S, KORONA-GLOWNIAK I, SKALICKA-WOZNIAK K, et al.. Phytochemical composition of wormwood (Artemisia gmelinii) extracts in respect of their antimicrobial activity [J/OL]. BMC Complementary Altern. Med., 2019,19(1):288 [2022-01-22]. . |
| 36 | YODA Y, HU Z Q, ZHAO W H, et al.. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate [J]. J. Infect. Chemother., 2004,10(1):55-58. |
| 37 | CHOI N, BAE Y, LEE S. Cell surface properties and biofilm formation of pathogenic bacteria [J]. Food Sci. Biotechnol., 2015,24(6):2257-2264. |
| 38 | LIU Y, HAN L, YANG H, et al.. Effect of apigenin on surface -associated characteristics and adherence of Streptococcus mutans [J]. Denal Materials J., 2020,39(6):933-940. |
| 39 | 萨仁高娃,胡文忠,冯可,等.植物精油及其成分对病原微生物抗菌机理的研究进展[J].食品科学, 2020,41(11):285-294. |
| Sarengaowa, HU W Z, FENG K, et al.. Antimicrobial mechanisms of essential oils and their components on pathogenic bacteria : a review [J]. Food Sci., 2020,41(11): 285-294. | |
| 40 | SONG Y J, YU H H, KIM Y J, et al.. Anti-biofilm activity of grapefruit seed extract against Staphylococcus aureus and Escherichia coli [J]. J. Microbiol. Biotechnol., 2019,29(8):1177-1183. |
| 41 | AFONINA I, LIM X N, TAN R, et al.. Planktonic interference and biofilm alliance between aggregation substance and endocarditis- and biofilm-associated pili in Enterococcus faecalis [J/OL]. J. Bacteriol., 2018,200(24):e00361 [2022-01-22]. . |
| 42 | 靳盼盼,刘亚文,邵美丽,等.香芹酚对食源粪肠球菌生物膜形成的抑制作用[J].中国食品学报, 2020,20(7):18-26. |
| JIN P P, LIU Y W, SHAO M L, et al.. Inhibition effect of carvacrol to biofilm formation of foodborne Enterococcus faecalis [J]. J. Chin. Inst. Food Sci. Technol., 2020,20(7): 18-26. | |
| 43 | LIU F, JIN P, GONG H, et al.. Antibacterial and antibiofilm activities of thyme oil against foodborne multiple antibiotics-resistant Enterococcus faecalis [J]. Poult. Sci., 2020,99(10):5127-5136. |
| [1] | Shegang SHAO, Ting LI, Yong LIU, Lanwen LIN, Dong ZHANG, Dong NI, Junjie LI, Li’an ZHU. Effects of Exogenous Promoting Bacteria Agent on Decomposition Characteristics and Microbial Community Structure of Rice Straw [J]. Journal of Agricultural Science and Technology, 2023, 25(9): 166-177. |
| [2] | Dongmeng ZHANG, Dongping YAO, Jun WU, Qiuhong LUO, Wen ZHUANG, Xionglun LIU, Qiyun DENG, Bin BAI. Effect of Natural Low Temperature on Cooking and Eating Quality of Rice During Grain Filling Stage [J]. Journal of Agricultural Science and Technology, 2023, 25(6): 144-153. |
| [3] | Lan MA, Qing PENG, Xiaoqing XU, Shuo YANG, Yuwei ZHANG, Dandan TIAN, Linbo SHI, Bo SHI, Yu QIAO. Gene Expression in Escherichia coli O157∶H7 Biofilms [J]. Journal of Agricultural Science and Technology, 2023, 25(6): 71-88. |
| [4] | WU Congmin, MA Lan, WU Yonghong, YU Yuanchun. Effects of Different Concentrations of Indole-3-acetic Acid on the Metabolic Characteristics of Microbial Communities in Periphyton [J]. Journal of Agricultural Science and Technology, 2021, 23(8): 74-79. |
| [5] | ZHOU Maochao1,2, HUANG Yanna2, DUAN Saifei1,2, SHU Shiyuan1,2, TANG Xueming2*. Development of Microbial Seed Coating Agents and Their Effects on the Growth of Maize Seedlings [J]. Journal of Agricultural Science and Technology, 2021, 23(4): 110-118. |
| [6] | SUI Fu1,2, LIU Xiaolin1,2, XIE Zhihong1,3*. Sensing Mechanism of Receptor TlpA1 to Succinic Acid in Azorhizobium caulinodans ORS571 [J]. Journal of Agricultural Science and Technology, 2020, 22(10): 77-84. |
| [7] | RAN Jiping1,2, YAN Yan2*, LI Cen 1, MAO Yutao3, XIAO Yu4, FENG Tingting5. Growth Conditions Optimization of Antagonistic Bacteria Against Powdery Mildew of Rosa roxburghii Tratt and Its Fermentation Broth Antibacterial Stability [J]. Journal of Agricultural Science and Technology, 2019, 21(11): 84-93. |
| [8] | WANG Dongfang, WANG Qingzhong, GAO Minggang. Insecticidal and Antibacterial Activity of Euphorbia esula Linn. Extract [J]. Journal of Agricultural Science and Technology, 2018, 20(8): 128-133. |
| [9] | GAO Yuan, WANG Fuling, JIA Qi, LIU Wei, ZHOU Yanyan, LI Jiali. Isolation and Identification of Endophytic Fungi from Taraxacum mongolicum Hand-Mazz and Antibacterial Activity Study [J]. Journal of Agricultural Science and Technology, 2017, 19(6): 55-60. |
| [10] | LI Yun1, LIU Xiaodong2, LI Qin2, ZHAN Yuhua1, YAN Yongliang1, LU Wei1*. The Regulatory Effect of RpoN on Flagellar Biosynthesis in Nitrogen-Fixing Pseudomonas stutzeri [J]. Journal of Agricultural Science and Technology, 2017, 19(11): 33-41. |
| [11] | GUAN Rufei, JIANG Ping, GAO Chao, XIA Lining*. Detection of Resistance and Drug Resistance Genes of Salmonella from the Chicken Farms Around Urumqi in Xinjiang [J]. Journal of Agricultural Science and Technology, 2017, 19(10): 28-35. |
| [12] | LIN Shuangshuang1,2, QIU Shanlian1, ZHENG Kaibin1,2*, LIU Huajian3, . Composition Analysis and Antibacterial Activity of the Essential Oil from Cymbopogon citratus [J]. Journal of Agricultural Science and Technology, 2017, 19(10): 89-95. |
| [13] | YANG Li-na1,2, ZHONG Juan1,2, ZHOU Jin-yan1*, TAN Hong1. Study on the Stability of the Fermentation Broth and Bioactive Metabolite Produced by Bionectria Ochroleuca YLZ42 [J]. Journal of Agricultural Science and Technology, 2016, 18(6): 72-79. |
| [14] | LU Jia-si1,2, SHANG Li-guo2, ZHAN Yu-hua2, LU Wei2, . Functional Analysis of bifA in Pseudomonas stutzeri [J]. Journal of Agricultural Science and Technology, 2016, 18(3): 67-73. |
| [15] | SHANG Li\|guo, ZHAN Yu\|hua, GONG Pai, YAN Yong\|liang*. Research Progress on the Function and Regulatory Mechanism of CsrA, a Global Regulator in Bacteria [J]. , 2014, 16(4): 79-86. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号