




















中国农业科技导报 ›› 2025, Vol. 27 ›› Issue (5): 21-38.DOI: 10.13304/j.nykjdb.2024.0502
收稿日期:2024-06-22
接受日期:2024-10-30
出版日期:2025-05-15
发布日期:2025-05-20
通讯作者:
石雅丽
作者简介:乔慧艳E-mail: qhy19980416@163.com;
基金资助:
Huiyan QIAO( ), Yali SHI(
), Yali SHI( ), Haojian HAN
), Haojian HAN
Received:2024-06-22
Accepted:2024-10-30
Online:2025-05-15
Published:2025-05-20
Contact:
Yali SHI
摘要:
开发可再生能源是解决目前能源需求逐年增加和温室气体排放的有效措施。可再生能源包括太阳能、水能、风能、生物质能、波浪能、潮汐能、海洋温差能、地热能等,它们在自然界可以循环再生。其中,生物质能源利用的途径之一是生物炼制,它是利用生物质降解后发电、制气、产乙醇等燃料。降解生物质的重要方法是利用纤维素酶对纤维素进行水解。目前,工业纤维素酶主要来源于微生物。从以下5个方面介绍了微生物来源纤维素酶的研究进展:①产纤维素酶的微生物,包括真菌、细菌和放线菌等;②微生物来源纤维素酶的特性,主要包括纤维素酶的分类及特性、纤维素酶结构以及催化机制;③纤维素酶活性的测定方法;④提高纤维素酶产量及活性的方法;⑤纤维素酶的应用。通过对微生物来源纤维素酶研究进展的阐述,为筛选和开发高效纤维素酶提供参考。
中图分类号:
乔慧艳, 石雅丽, 韩昊健. 微生物来源纤维素酶的研究进展[J]. 中国农业科技导报, 2025, 27(5): 21-38.
Huiyan QIAO, Yali SHI, Haojian HAN. Research Progress of Cellulase Derived from Microorganisms[J]. Journal of Agricultural Science and Technology, 2025, 27(5): 21-38.
菌种类 Species of strain | 来源 Source | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献Reference |
|---|---|---|---|---|
| 甲基营养型芽孢杆菌Bacillus methylotrophicus | 玉米地土壤 Corn field soil | 4.0~5.0 | 50 | [ |
| 枯草芽孢杆菌Bacillus subtilis | 玉米地土壤 Corn field soil | 4.0~5.0 | 50 | [ |
腐木 Rotten wood | 7.0 | 34 | [ | |
| 地衣芽孢杆菌Bacillus licheniformis | 汤池温泉水 Hot spring water of bathing pool | 7.0 | 73 | [ |
| 解淀粉芽孢杆菌Bacillus amyloliquefaciens | 山羊瘤胃内容物 Goat rumen contents | 7.0 | 35 | [ |
堆肥 Compost | 6.0 | 34 | [ | |
| 嗜热芽孢杆菌Bacillus thermoleovorans | 土壤 Soil | 9.0 | 70 | [ |
| 蜡样芽孢杆菌Bacillus cereus | 瘤胃 Rumen | 6.0 | 40 | [ |
| 巨大芽孢杆菌Bacillus megaterium | 玉米地土壤 Corn field soil | 6.0 | 50 | [ |
| 贝莱斯芽孢杆菌Bacillus velezensis | 土壤 Soil | 6.0 | 50 | [ |
| 短小芽孢杆菌Bacillus pumilus | 高温期堆肥 Composting during high temperature period | — | 55~65 | [ |
| 暹罗芽孢杆菌Bacillus siamensis | 海鱼肠道 Intestine of marine fish | 8.5 | 30 | [ |
| 苏云金芽孢杆菌Bacillus thuringiensis | 腐殖土壤 Humus soil | 7.0~7.2 | 30 | [ |
表1 芽孢杆菌的种类及其产纤维素酶特性
Table 1 Types of Bacillus and their cellulase production characteristics
菌种类 Species of strain | 来源 Source | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献Reference |
|---|---|---|---|---|
| 甲基营养型芽孢杆菌Bacillus methylotrophicus | 玉米地土壤 Corn field soil | 4.0~5.0 | 50 | [ |
| 枯草芽孢杆菌Bacillus subtilis | 玉米地土壤 Corn field soil | 4.0~5.0 | 50 | [ |
腐木 Rotten wood | 7.0 | 34 | [ | |
| 地衣芽孢杆菌Bacillus licheniformis | 汤池温泉水 Hot spring water of bathing pool | 7.0 | 73 | [ |
| 解淀粉芽孢杆菌Bacillus amyloliquefaciens | 山羊瘤胃内容物 Goat rumen contents | 7.0 | 35 | [ |
堆肥 Compost | 6.0 | 34 | [ | |
| 嗜热芽孢杆菌Bacillus thermoleovorans | 土壤 Soil | 9.0 | 70 | [ |
| 蜡样芽孢杆菌Bacillus cereus | 瘤胃 Rumen | 6.0 | 40 | [ |
| 巨大芽孢杆菌Bacillus megaterium | 玉米地土壤 Corn field soil | 6.0 | 50 | [ |
| 贝莱斯芽孢杆菌Bacillus velezensis | 土壤 Soil | 6.0 | 50 | [ |
| 短小芽孢杆菌Bacillus pumilus | 高温期堆肥 Composting during high temperature period | — | 55~65 | [ |
| 暹罗芽孢杆菌Bacillus siamensis | 海鱼肠道 Intestine of marine fish | 8.5 | 30 | [ |
| 苏云金芽孢杆菌Bacillus thuringiensis | 腐殖土壤 Humus soil | 7.0~7.2 | 30 | [ |
菌种类 Species of strain | 纤维素酶类型 Cellulase type | 酶活力 Enzyme activity/(U·mL-1) | 最适 pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献Reference |
|---|---|---|---|---|---|
深红紫链霉菌 Streptomyces violaceorubidus | 内切纤维素酶 Endoglucanase | 289.0 | — | 50 | [ |
纤维素酶(滤纸酶活) Cellulase (filter paper enzyme activity) | 281.0 | ||||
β-葡萄糖苷酶 β-glucosidase | 271.0 | ||||
嗜热链球菌Fx-1 Thermophilus Fx-1 | — | — | 7.0 | 60 | [ |
钉斑链霉菌 Streptomyces clavifer | 内切纤维素酶 Endoglucanase | 21.6 | 6.0 | 40 | [ |
嗜热一氧化碳链霉菌 Streptomyces thermophilus | 纤维素酶(滤纸酶活) Cellulase (filter paper enzyme activity) | 135.5 | — | — | [ |
链霉菌ND2-1 Streptomyces ND2-1 | 纤维素酶 Cellulase | 122.0 | 7.0 | 25 | [ |
远青链霉菌T23-B Streptomyces azureus T23-B | 纤维素酶 Cellulase | 123.4 | — | 15 | [ |
表2 放线菌的种类及其产纤维素酶特性
Table 2 Types of Actinomycetes and characteristics of cellulase production
菌种类 Species of strain | 纤维素酶类型 Cellulase type | 酶活力 Enzyme activity/(U·mL-1) | 最适 pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献Reference |
|---|---|---|---|---|---|
深红紫链霉菌 Streptomyces violaceorubidus | 内切纤维素酶 Endoglucanase | 289.0 | — | 50 | [ |
纤维素酶(滤纸酶活) Cellulase (filter paper enzyme activity) | 281.0 | ||||
β-葡萄糖苷酶 β-glucosidase | 271.0 | ||||
嗜热链球菌Fx-1 Thermophilus Fx-1 | — | — | 7.0 | 60 | [ |
钉斑链霉菌 Streptomyces clavifer | 内切纤维素酶 Endoglucanase | 21.6 | 6.0 | 40 | [ |
嗜热一氧化碳链霉菌 Streptomyces thermophilus | 纤维素酶(滤纸酶活) Cellulase (filter paper enzyme activity) | 135.5 | — | — | [ |
链霉菌ND2-1 Streptomyces ND2-1 | 纤维素酶 Cellulase | 122.0 | 7.0 | 25 | [ |
远青链霉菌T23-B Streptomyces azureus T23-B | 纤维素酶 Cellulase | 123.4 | — | 15 | [ |
酶类型 Enzyme type | 名称 Name | EC号 EC number | CAZy家族 CAZy family | 底物类型 Substrate type | 作用位点 Site of action | 降解产物 Degradation product |
|---|---|---|---|---|---|---|
外切 纤维素酶 Exoglucanase | 外切β-1,4葡聚糖酶、纤维二糖水解酶、 C1纤维素酶 Exo-1,4-β-D-glucanase, cellobiohydrolase, C1 cellulase | EC3.2.1.74 | GH1, GH3, GH5, GH9, GH39 | 无定向 纤维素、 结晶纤维素 Undirected cellulose, crystalline cellulose | 纤维素链末端(还原端或非还原端) Cellulose chain ends (nonreducing end or nonreducing end) | 葡萄糖、 纤维二糖 Glucose, cellobiose |
| EC3.2.1.91 | GH5, GH6, GH9, GH48 | |||||
| EC3.2.1.176 | GH7, GH48 | |||||
内切 纤维素酶 Endoglucanase | 内切-β-1,4-葡聚糖酶、羧甲基纤维素酶、CX纤维素酶 Endo-1,4-β-D-glucanase,carboxymethylcellulase, CX cellulase | EC3.2.1.4 | GH5, GH6, GH7, GH8, GH9, GH10, GH12, GH26, GH44, GH45, GH48, GH51, GH124, GH148 | 结晶纤维素、 无定形纤维素 Crystalline cellulose, amorphous cellulose | 纤维素链内部(无定形区) Inside the cellulose chain (amorphous region) | 纤维二糖 Cellobiose |
β-葡萄糖 苷酶 β-glucosidase | 纤维二糖酶、 芳基-β-葡糖苷酶 Cellobiase, aryl-β-glucosidase | EC3.2.1.21 | GH1, GH2, GH3, GH5, GH16, GH30, GH39, GH116, GH131 | 纤维二糖、 纤维寡糖 Cellobiose, cello-oligosaccharides | 非还原端 Nonreducing end | 葡萄糖 Glucose |
表3 纤维素酶的种类及特点
Table 3 Types and characteristics of cellulase
酶类型 Enzyme type | 名称 Name | EC号 EC number | CAZy家族 CAZy family | 底物类型 Substrate type | 作用位点 Site of action | 降解产物 Degradation product |
|---|---|---|---|---|---|---|
外切 纤维素酶 Exoglucanase | 外切β-1,4葡聚糖酶、纤维二糖水解酶、 C1纤维素酶 Exo-1,4-β-D-glucanase, cellobiohydrolase, C1 cellulase | EC3.2.1.74 | GH1, GH3, GH5, GH9, GH39 | 无定向 纤维素、 结晶纤维素 Undirected cellulose, crystalline cellulose | 纤维素链末端(还原端或非还原端) Cellulose chain ends (nonreducing end or nonreducing end) | 葡萄糖、 纤维二糖 Glucose, cellobiose |
| EC3.2.1.91 | GH5, GH6, GH9, GH48 | |||||
| EC3.2.1.176 | GH7, GH48 | |||||
内切 纤维素酶 Endoglucanase | 内切-β-1,4-葡聚糖酶、羧甲基纤维素酶、CX纤维素酶 Endo-1,4-β-D-glucanase,carboxymethylcellulase, CX cellulase | EC3.2.1.4 | GH5, GH6, GH7, GH8, GH9, GH10, GH12, GH26, GH44, GH45, GH48, GH51, GH124, GH148 | 结晶纤维素、 无定形纤维素 Crystalline cellulose, amorphous cellulose | 纤维素链内部(无定形区) Inside the cellulose chain (amorphous region) | 纤维二糖 Cellobiose |
β-葡萄糖 苷酶 β-glucosidase | 纤维二糖酶、 芳基-β-葡糖苷酶 Cellobiase, aryl-β-glucosidase | EC3.2.1.21 | GH1, GH2, GH3, GH5, GH16, GH30, GH39, GH116, GH131 | 纤维二糖、 纤维寡糖 Cellobiose, cello-oligosaccharides | 非还原端 Nonreducing end | 葡萄糖 Glucose |
来源 Source | GenBank编号 GenBank number | 外切纤维素酶 Exoglucanase | 相对分子质量 Relative molecular mass/ku | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|---|---|
里氏木霉 Trichoderma reesei | — | CBH Ⅰ,CBH Ⅱ | — | 5.0 | 常温 Room temperature | [ |
绿色木霉 Trichoderma viride | — | CBH Ⅰ,CBH Ⅱ | 65 | 5.5 | 45 | [ |
康氏木霉 Trichoderma koningii | — | CBH Ⅰ | — | 4.5~5.0 | 45~50 | [ |
草酸青霉 Penicillium oxalate | HQ843504 | CBH Ⅰ | — | 6.0 | 55 | [ |
| — | CBH Ⅰ | 57 | 6.0 | 55 | [ | |
斜卧青霉 Penicillium decumbens | EF397602 | CBH Ⅰ | 53, 52, 57, 60, 47 | 7.4 | — | [ |
蝇状青霉 Penicillium muscariforme | AJ312295 | CBH Ⅰ | 46 | 4.4 | — | [ |
黑曲霉Asp-524 Aspergillus niger Asp-524 | — | CBH B | 57 | 5.0 | 50 | [ |
地衣芽孢杆菌 Bacillus licheniformis | AAU40776.1 | Cel A | 79 | 9.0 | 50 | [ |
短小芽孢杆菌 Bacillus pumilus | — | CBH Ⅱ | 78 | 9.5 | 50 | [ |
嗜热毁丝菌 Myceliophthora thermophila | — | CBH Ⅰ | 72 | 5.0 | 50 | [ |
表4 典型的外切纤维素酶的特性
Table 4 Characteristics of typical exoglucanase
来源 Source | GenBank编号 GenBank number | 外切纤维素酶 Exoglucanase | 相对分子质量 Relative molecular mass/ku | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|---|---|
里氏木霉 Trichoderma reesei | — | CBH Ⅰ,CBH Ⅱ | — | 5.0 | 常温 Room temperature | [ |
绿色木霉 Trichoderma viride | — | CBH Ⅰ,CBH Ⅱ | 65 | 5.5 | 45 | [ |
康氏木霉 Trichoderma koningii | — | CBH Ⅰ | — | 4.5~5.0 | 45~50 | [ |
草酸青霉 Penicillium oxalate | HQ843504 | CBH Ⅰ | — | 6.0 | 55 | [ |
| — | CBH Ⅰ | 57 | 6.0 | 55 | [ | |
斜卧青霉 Penicillium decumbens | EF397602 | CBH Ⅰ | 53, 52, 57, 60, 47 | 7.4 | — | [ |
蝇状青霉 Penicillium muscariforme | AJ312295 | CBH Ⅰ | 46 | 4.4 | — | [ |
黑曲霉Asp-524 Aspergillus niger Asp-524 | — | CBH B | 57 | 5.0 | 50 | [ |
地衣芽孢杆菌 Bacillus licheniformis | AAU40776.1 | Cel A | 79 | 9.0 | 50 | [ |
短小芽孢杆菌 Bacillus pumilus | — | CBH Ⅱ | 78 | 9.5 | 50 | [ |
嗜热毁丝菌 Myceliophthora thermophila | — | CBH Ⅰ | 72 | 5.0 | 50 | [ |
来源 Source | 内切纤维素酶 Endoglucanase | 相对分子质量 Relative molecular weight/ku | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|---|
里氏木霉 Trichoderma reesei | EG Ⅱ | — | 5.0 | 55 | [ |
绿色木霉 Trichoderma viride | EG Ⅳ | 26.8 | 6.4 | 60 | [ |
康氏木霉 Trichoderma koningii | EG Ⅰ | 45.0 | 6.0 | 30~40 | [ |
大肠杆菌ZH-4 Escherichia coli ZH-4 | BcsZ | 41.7 | 6.0 | 50 | [ |
黑曲霉 Aspergillus niger | En4gA | — | 3.5~6.0 | 45~65 | [ |
| En4gG | 2.0~7.0 | 40~80 | |||
嗜热梭菌 Clostridium thermophilum | Cel D | 72.4 | 6.0 | 50 | [ |
乳酸菌 Lactobacillus | EG | 50.0 | 6.0 | 90 | [ |
表5 典型的内切纤维素酶的特性
Table 5 Characteristics of typical endoglucanase
来源 Source | 内切纤维素酶 Endoglucanase | 相对分子质量 Relative molecular weight/ku | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|---|
里氏木霉 Trichoderma reesei | EG Ⅱ | — | 5.0 | 55 | [ |
绿色木霉 Trichoderma viride | EG Ⅳ | 26.8 | 6.4 | 60 | [ |
康氏木霉 Trichoderma koningii | EG Ⅰ | 45.0 | 6.0 | 30~40 | [ |
大肠杆菌ZH-4 Escherichia coli ZH-4 | BcsZ | 41.7 | 6.0 | 50 | [ |
黑曲霉 Aspergillus niger | En4gA | — | 3.5~6.0 | 45~65 | [ |
| En4gG | 2.0~7.0 | 40~80 | |||
嗜热梭菌 Clostridium thermophilum | Cel D | 72.4 | 6.0 | 50 | [ |
乳酸菌 Lactobacillus | EG | 50.0 | 6.0 | 90 | [ |
来源 Source | β-葡萄糖苷酶 β-glucosidase | 相对分子质量 Relative molecular weight/ku | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|---|
里氏木霉 Trichoderma reesei | BGL | 57 | 7.0 | 30 | [ |
瑞氏木霉 Trichoderma Swiss | BGL Ⅰ, BGL Ⅱ | 75, 52, 94, 91 | 5.0 | 70 | [ |
草酸青霉 Penicillium oxalate | BGL 1, BGL 4, BGL 5 | — | 5.0 | — | [ |
斜卧青霉 Penicillium decumbens | BGL | 310 | 7.0 | 65 | [ |
黑曲霉 Aspergillus niger | BGL | — | 5.6 | 40 | [ |
枯草芽孢杆菌 Bacillus subtilis | TpBgl3 | — | 4.0 | 85 | [ |
娄彻氏链霉菌 Streptomycete rochei | Egl | — | 8.0 | 40 | [ |
表6 典型的β-葡萄糖苷酶的特性
Table 6 Characteristics of typical β-glucosidase
来源 Source | β-葡萄糖苷酶 β-glucosidase | 相对分子质量 Relative molecular weight/ku | 最适pH Optimum pH | 最适温度 Optimum temperature/℃ | 参考文献 Reference |
|---|---|---|---|---|---|
里氏木霉 Trichoderma reesei | BGL | 57 | 7.0 | 30 | [ |
瑞氏木霉 Trichoderma Swiss | BGL Ⅰ, BGL Ⅱ | 75, 52, 94, 91 | 5.0 | 70 | [ |
草酸青霉 Penicillium oxalate | BGL 1, BGL 4, BGL 5 | — | 5.0 | — | [ |
斜卧青霉 Penicillium decumbens | BGL | 310 | 7.0 | 65 | [ |
黑曲霉 Aspergillus niger | BGL | — | 5.6 | 40 | [ |
枯草芽孢杆菌 Bacillus subtilis | TpBgl3 | — | 4.0 | 85 | [ |
娄彻氏链霉菌 Streptomycete rochei | Egl | — | 8.0 | 40 | [ |
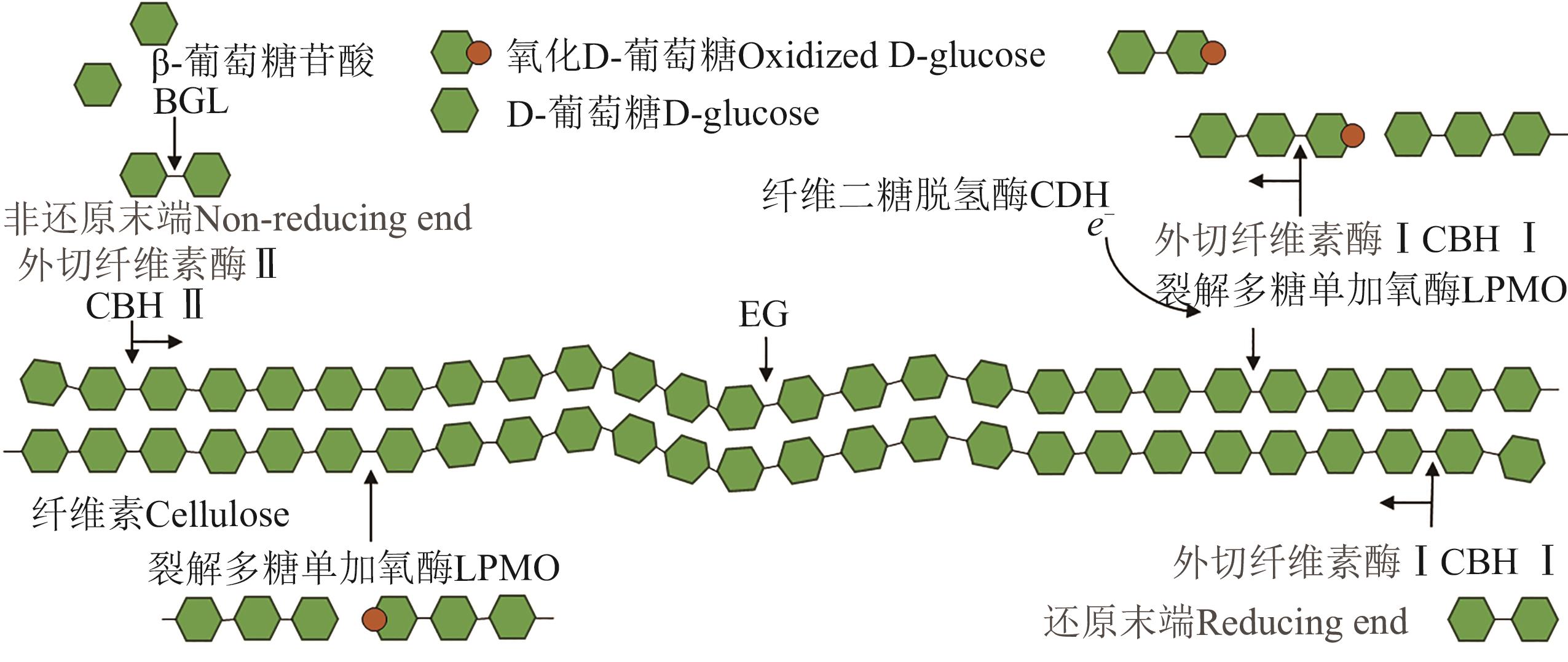
图1 纤维二糖脱氢酶和裂解多糖单加氧酶与纤维素酶结合参与纤维素降解[75]
Fig. 1 Cellobiose dehydrogenase and lytic polysaccharide monooxygenases bind with cellulase to participate in cellulose degradation[75]
测定方法 Measurement method | 酶 Enzyme | 底物 Substrate | 原理 Principle | 优点 Advantage |
|---|---|---|---|---|
荧光微纤维 Fluorescent microfibrils | 内切葡聚糖酶、外切葡聚糖酶 Endoglucanase, exoglucanase | 细菌纤维素 Bacterial cellulose | 当纤维素酶作用于微纤维时,会引发特定的化学反应,释放出荧光信号,通过检测荧光信号的强度或变化,间接反映纤维素酶的活性 When cellulase acts on microfibers, it triggers a specific chemical reaction, releasing a fluorescent signal. By detecting the intensity or change of the fluorescent signal, it indirectly reflects the activity of cellulase | 可重复、高通量及减少试剂用量 Reproducible, high-throughput and reduced reagent usage |
微型比色法 Miniaturized colorimetric assay | 内切纤维素酶,外切纤维素酶, β-葡萄糖苷酶 Endoglucanase, exocellulose, β-glucosidase | 滤纸 Filter paper | 利用化学反应产生的颜色变化来定量测定物质的含量或活性 Quantitatively determine the content or activity of a substance by utilizing the color changes generated by chemical reactions | 灵敏、高通量 Sensitive and high-throughput |
羧甲基纤维素钠 CMC | 高通量、可重复及减少试剂用量 High-throughput, repeatable and reduced reagent usage | |||
内切纤维素酶 Endoglucanase | ||||
纤维素酶 Cellulase | 微晶纤维素、麦秆和柳枝稷等 Avicel, corn stalk, switchgrass, arabinoxylan | 高通量、底物多样及减少试剂用量 High-throughput, diverse substrates and reduced reagentusage | ||
重量分析法 Gravimetric analysis | 纤维素酶 Cellulase | 醋酸纤维素薄膜 Cellulose acetate films | 基于测定酶解反应前后底物或产物的质量变化 Based on measuring the quality changes of substrates or products before and after enzymatic hydrolysis reaction | 灵敏度高、效率高 High sensitivity and efficiency |
滤纸折叠法 Filter paper collapsing method | 滤纸酶 FPA | 滤纸 Filter paper | 通过将滤纸折叠成一定形状并浸泡在含有纤维素酶的溶液中,观察滤纸被降解的程度评估酶活性的高低 By folding the filter paper into a certain shape and soaking it in a solution containing cellulase, observe the degree of degradation of the filter paper to evaluate the level of enzyme activity | 高灵敏度高、快捷筛选、操作简单及成本低 High sensitivity, quick screening, simple operation and low cost |
荧光共振能量转移 Fluorescence resonance energy transfer | 内切纤维素酶 Endoglucanase | 5-氨基甲基荧光素标记的羧甲基纤维素 Labeled CMC with 5-aminomethyl fluorescein | 基于荧光现象的分子间相互作用 Molecular interactions based on fluorescence phenomenon | 快速实时记录、 高度灵敏 Fast real-time recording and highly sensitive |
石英晶体微量天平 Quartz crystal microbalance dissipiation | 纤维素酶 Cellulase | 滤纸、微晶纤维素、羧甲基纤维素钠、木质纤维素纳米纤维薄膜 FP, Avicel, CMC, lignocellulosic nanofibrils (LCNFs) films | 将纤维素酶固定在石英晶体表面,通过监测酶解反应过程中晶体表面质量的变化来反映酶活性 Fix cellulase on the surface of quartz crystals and monitor the changes in crystal surface quality during enzymatic hydrolysis to reflect the level of enzyme activity | 底物多样、操作简单 Diverse substrates and simple operation |
表7 测定纤维素酶活性的新方法
Table 7 New methods for determining cellulase activity
测定方法 Measurement method | 酶 Enzyme | 底物 Substrate | 原理 Principle | 优点 Advantage |
|---|---|---|---|---|
荧光微纤维 Fluorescent microfibrils | 内切葡聚糖酶、外切葡聚糖酶 Endoglucanase, exoglucanase | 细菌纤维素 Bacterial cellulose | 当纤维素酶作用于微纤维时,会引发特定的化学反应,释放出荧光信号,通过检测荧光信号的强度或变化,间接反映纤维素酶的活性 When cellulase acts on microfibers, it triggers a specific chemical reaction, releasing a fluorescent signal. By detecting the intensity or change of the fluorescent signal, it indirectly reflects the activity of cellulase | 可重复、高通量及减少试剂用量 Reproducible, high-throughput and reduced reagent usage |
微型比色法 Miniaturized colorimetric assay | 内切纤维素酶,外切纤维素酶, β-葡萄糖苷酶 Endoglucanase, exocellulose, β-glucosidase | 滤纸 Filter paper | 利用化学反应产生的颜色变化来定量测定物质的含量或活性 Quantitatively determine the content or activity of a substance by utilizing the color changes generated by chemical reactions | 灵敏、高通量 Sensitive and high-throughput |
羧甲基纤维素钠 CMC | 高通量、可重复及减少试剂用量 High-throughput, repeatable and reduced reagent usage | |||
内切纤维素酶 Endoglucanase | ||||
纤维素酶 Cellulase | 微晶纤维素、麦秆和柳枝稷等 Avicel, corn stalk, switchgrass, arabinoxylan | 高通量、底物多样及减少试剂用量 High-throughput, diverse substrates and reduced reagentusage | ||
重量分析法 Gravimetric analysis | 纤维素酶 Cellulase | 醋酸纤维素薄膜 Cellulose acetate films | 基于测定酶解反应前后底物或产物的质量变化 Based on measuring the quality changes of substrates or products before and after enzymatic hydrolysis reaction | 灵敏度高、效率高 High sensitivity and efficiency |
滤纸折叠法 Filter paper collapsing method | 滤纸酶 FPA | 滤纸 Filter paper | 通过将滤纸折叠成一定形状并浸泡在含有纤维素酶的溶液中,观察滤纸被降解的程度评估酶活性的高低 By folding the filter paper into a certain shape and soaking it in a solution containing cellulase, observe the degree of degradation of the filter paper to evaluate the level of enzyme activity | 高灵敏度高、快捷筛选、操作简单及成本低 High sensitivity, quick screening, simple operation and low cost |
荧光共振能量转移 Fluorescence resonance energy transfer | 内切纤维素酶 Endoglucanase | 5-氨基甲基荧光素标记的羧甲基纤维素 Labeled CMC with 5-aminomethyl fluorescein | 基于荧光现象的分子间相互作用 Molecular interactions based on fluorescence phenomenon | 快速实时记录、 高度灵敏 Fast real-time recording and highly sensitive |
石英晶体微量天平 Quartz crystal microbalance dissipiation | 纤维素酶 Cellulase | 滤纸、微晶纤维素、羧甲基纤维素钠、木质纤维素纳米纤维薄膜 FP, Avicel, CMC, lignocellulosic nanofibrils (LCNFs) films | 将纤维素酶固定在石英晶体表面,通过监测酶解反应过程中晶体表面质量的变化来反映酶活性 Fix cellulase on the surface of quartz crystals and monitor the changes in crystal surface quality during enzymatic hydrolysis to reflect the level of enzyme activity | 底物多样、操作简单 Diverse substrates and simple operation |
| 1 | KONAR A, AICH S, KATAKOJWALA R, et al.. A processive GH9 family endoglucanase of Bacillus licheniformis and the role of its carbohydrate-binding domain [J]. Appl. Microbiol. Biotechnol., 2022, 106(18):6059-6075. |
| 2 | WU Z Y, PENG K, ZHANG Y, et al.. Lignocellulose dissociation with biological pretreatment towards the biochemical platform: a review [J/OL]. Mater. Today Biol, 2022, 16:100445 [2024-05-20]. . |
| 3 | CHANDRA M R G S, MADAKKA M. Comparative biochemistry and kinetics of microbial lignocellulolytic enzymes [M]// Recent developments in applied microbiology and biochemistry. Academic Press, 2019: 147-159. |
| 4 | SJULANDER N, KIKAS T. Two-step pretreatment of lignocellulosic biomass for high-sugar recovery from the structural plant polymers cellulose and hemicellulose [J]. Energies, 2022, 15(23): 8898-8905. |
| 5 | EL H M, RAJHA H N, MAACHE-REZZOUG Z, et al.. Intensification of bioethanol production from different lignocellulosic biomasses, induced by various pretreatment methods: an updated review [J]. Energies, 2022, 15(19): 6912-6925. |
| 6 | ILIC N, MILIC M, BELUHAN S, et al.. Cellulases: from lignocellulosic biomass to improved production [J]. Energies, 2023, 16(8): 3598-3604. |
| 7 | MONCLARO A V, DE OLIVEIRA GORGULHO SILVA C, GOMES H A R, et al.. The enzyme interactome concept in filamentous fungi linked to biomass valorization [J/OL]. Bioresour. Technol., 2022, 344 (Pt A): 126200 [2024-05-20]. . |
| 8 | 张玢.瑞氏木霉CBHⅠ的结构与糖苷合成性质的探讨[D].南京:南京师范大学,2007. |
| 9 | 赵萱,顿宝庆,顾金刚,等.草酸青霉外切葡聚糖酶基因(cbh1)克隆及其在毕赤酵母中的表达和重组外切葡聚糖酶特性分析[J].农业生物技术学报,2011,19(6):1127-1135. |
| ZHAO X, DUN B Q, GU J G, et al.. Cloning of an exo-β-1, 4-D-glucanases gene (cbh1) from Penicillium oxalicum, its expression in Pichia pastoris and characterization of recombination CBHⅠ [J]. J. Agric. Biotechnol., 2011, 19(6): 1127-1135. | |
| 10 | 赵君. 黑曲霉胞内β-葡萄糖苷酶的鉴定及其在纤维素降解酶系合成调控中的作用[D]. 郑州: 河南农业大学, 2021. |
| ZHAO J. Identification of intracellular β-glucosidase in Aspergillus niger and its role in the regulation of lignocellulose-degrading enzyme synthesis [D]. Zhengzhou: Henan Agricultural University, 2021. | |
| 11 | SUKUMARAN R K, CHRISTOPHER M, KOOLOTH-VALAPPIL P, et al.. Addressing challenges in production of cellulases for biomass hydrolysis:targeted interventions into the genetics of cellulase producing fungi [J/OL]. Bioresour. Technol., 2021, 329:12474610 [2024-05-20]. . |
| 12 | CABRERA I E, OZA Y, CARRILLO A J, et al.. Regulator of G protein signaling proteins control growth,development and cellulase production in Neurospora crassa [J/OL]. J. Fungi (Basel), 2022, 8(10):1076 [2024-05-20]. . |
| 13 | FAHEINA JUNIOR G S, SOUSA K A, ZILLI J E, et al.. Enhanced cellulase production by Talaromyces amestolkiae CMIAT055 using banana pseudostem [J].Waste Biomass Valorization, 2022, 13(8):3535-3546. |
| 14 | VASCO-CORREA J, CAPOUYA R, SHAH A, et al.. Sequential fungal pretreatment of unsterilized Miscanthus: changes in composition,cellulose digestibility and microbial communities [J]. Appl. Microbiol. Biotechnol., 2022, 106(5/6):2263-2279. |
| 15 | 王丰园,金海炎,丁凌飞,等.纤维素酶及其活性提升研究进展[J].现代农村科技,2022(3):65-68. |
| 16 | 李喜海.丝状真菌里氏木霉异源表达Insulin Glargine的初步研究[D].济南:山东大学,2021. |
| LI X H. A preliminary study on the heterologous expression of insulin glargine by the filamentous fungus Trichoderma reesei [D]. Ji'nan: Shandong University, 2021. | |
| 17 | 卢敏.康氏木霉纤维素酶CBH I基因克隆及在毕赤酵母中的表达[D].郑州:河南农业大学,2012. |
| LU M. Cloning of cellulase CBH I gene from Trichoderma koningii and its expression in Pichia pastoris [D]. Zhengzhou: Henan Agricultural University, 2012. | |
| 18 | 刘相致,程驰,赵悦,等.里氏木霉中纤维素酶的合成诱导及调控[J].中国生物工程杂志,2022,42(10):93-104. |
| LIU X Z, CHENG C, ZHAO Y, et al.. Induction and regulation of cellulase synthesis in Trichoderma reesei [J]. China Biotechnol., 2022, 42(10):93-104. | |
| 19 | MISHRA A, PANDEY S, TIWARI S, et al.. Trichoderma spp.: versatile biocontrol agents and their impact on plant growth and soil health [J/OL]. J. Fungi, 2023, 9(10):826 [2024-05-20].. |
| 20 | 李志刚.草酸青霉纤维素酶表达调控因子的挖掘及功能研究[D].济南:齐鲁工业大学,2021. |
| LI Z G. Mining and functional study on the regulator of cellulase expression in Penicillium oxalicum [D]. Jinan: Qilu University of Technology, 2021. | |
| 21 | 刘国栋,高丽伟,曲音波.青霉生产木质纤维素降解酶系的研究进展[J].生物工程学报,2021,37(3):1058-1069. |
| LIU G D, GAO L W, QU Y B. Progress in the production of lignocellulolytic enzyme systems using Penicillium species [J]. Chin. J. Biotechnol., 2021, 37(3):1058-1069. | |
| 22 | 梁倩,李荷,王卓娅.产纤维素酶细菌的分离、鉴定与酶学性质研究[J].广东药科大学学报,2019,35(1):120-125. |
| LIANG Q, LI H, WANG Z Y. Isolation and identification of cellulase-producing strains and characterization of their cellulases [J]. J. Guangdong Pharm. Univ., 2019, 35(1):120-125. | |
| 23 | SHAHID S, TAJWAR R, AKHTAR M W. A novel trifunctional, family GH10 enzyme from Acidothermus cellulolyticus 11B, exhibiting endo-xylanase, arabinofuranosidase and acetyl xylan esterase activities [J]. Extremophiles, 2018, 22(1):109-119. |
| 24 | 毛婷,朱瑞清,牛永艳,等.纤维素降解芽孢菌的筛选及产酶条件优化[J].中国酿造,2020,39(1):71-76. |
| MAO T, ZHU R Q, NIU Y Y, et al.. Screening and enzyme production conditions optimization of cellulose-degrading Bacillus [J]. China Brew., 2020, 39(1):71-76. | |
| 25 | 张凤琴.一株嗜热地衣芽孢杆菌的分离及产纤维素酶特性研究[J].巢湖学院学报,2021,23(6):8-14, 30. |
| ZHANG F Q. Isolation and cellulase production characteristics of one thermophilic Geobacillus [J]. J. Chaohu Univ., 2021, 23(6):8-14, 30. | |
| 26 | 徐同.一株产纤维素酶瘤胃源微生物特性的研究[D].大连:大连工业大学,2019. |
| XU T. Study on the characteristics of microorganisms producing cellulase derived from rumen [D]. Dalian: Dalian University of Technology, 2019. | |
| 27 | 杨艳红,刘旸,张浩铂,等.一株产纤维素酶细菌B4的鉴定及发酵条件优化[J].重庆理工大学学报(自然科学版),2022,36(5):304-311. |
| 28 | 朱檬,刘国瑞,张军,等.一株喜油嗜热芽孢杆菌G1201产高温蛋白酶的性质研究及异源表达初探[J].中国饲料,2022(9):30-37. |
| ZHU M, LIU G R, ZHANG J, et al.. Characterization and heterologous expression of a thermophilic protease produced by Geobacillus thermoleovorans G1201 [J]. China Feed., 2022(9):30-37. | |
| 29 | 韦海婷,刘晗璐,李光玉,等.梅花鹿瘤胃纤维素降解菌的分离鉴定及其酶活力测定[J].中国畜牧兽医,2018,45(4):888-897. |
| WEI H T, LIU H L, LI G Y, et al.. Isolation,identification and determinations of cellulose-degrading bacteria from Sika deer (Cervus nippon) rumen [J]. China Anim. Husb. Vet. Med., 2018, 45(4):888-897. | |
| 30 | 赵龙妹,陈林,杜东晓,等.产纤维素酶细菌的筛选鉴定与特性分析[J].中国农学通报,2021,37(30):83-88. |
| ZHAO L M, CHEN L, DU D X, et al.. Screening,identification and characteristic analysis of cellulase-producing bacteria [J]. Chin. Agric. Sci. Bull., 2021, 37(30):83-88. | |
| 31 | 孙会刚,徐慧敏,黄天姿,等.产酸性纤维素酶细菌的筛选鉴定及其酶学性质[J].食品科技,2021,46(7):55-59. |
| SUN H G, XU H M, HUANG T Z, et al.. Screening,isolating and enzymatic properties of bacterium producing acidic cellulase [J]. Food Sci. Technol., 2021, 46(7):55-59. | |
| 32 | 江高飞,暴彦灼,杨天杰,等.高温秸秆降解菌的筛选及其纤维素酶活性研究[J].农业环境科学学报,2020,39(10):2465-2472. |
| JIANG G F, BAO Y Z, YANG T J, et al.. Screening of thermophilic cellulolytic bacteria and investigation of cellulase thermostability [J]. J. Agro-Environ. Sci., 2020, 39(10):2465-2472. | |
| 33 | 沙沙,刘心怡,张玉林,等.一株产纤维素酶的暹罗芽孢杆菌筛选及产酶条件优化[J].河南科学,2019,37(7):1073-1081. |
| SHA S, LIU X Y, ZHANG Y L, et al.. UV mutagenesis and fermentation conditions optimization of a cellulase-producing marine Bacillus siamensis [J]. Henan Sci., 2019, 37(7):1073-1081. | |
| 34 | 周思才,石家城,高瑞佳,等.一株纤维素降解菌的分离和鉴定[J].西昌学院学报(自然科学版),2020,34(4):5-7, 27. |
| ZHOU S C, SHI J C, GAO R J, et al.. Isolation and identification of a cellulolytic Bacterium [J]. J. Xichang Univ. (Nat. Sci.), 2020, 34(4): 5-7, 27. | |
| 35 | SUDARSHAN A, RENUKA S, RESHAM S, et al.. Upsurge production of cellulase from maize stover under solid state conditions mediated by Streptomyces enissocaesilis DQ026641 [J]. J. Appl. Biol. Biotechnol., 2022, 79(1): 136-144. |
| 36 | 付晓微.产耐高温纤维素酶放线菌的筛选与鉴定及菌株发酵条件优化[D].哈尔滨:东北农业大学,2015. |
| FU X W. Screening and identification of cellulase-producing actinomycetes with the capacity of high-temperature tolerance and optimization of its fermentation conditions [D]. Harbin: Northeast Agricultural University, 2015. | |
| 37 | 魏越波,郭晓军,孙悦龙,等.发酵用高温放线菌的筛选及性质研究[J].饲料工业,2020,41(21):47-52. |
| WEI Y B, GUO X J, SUN Y L, et al.. Screening and properties of high-temperature actinomycetes for fermentation [J]. Feed. Ind., 2020, 41(21):47-52. | |
| 38 | EL-SAYED M H, ELSAYED D A, GOMAA A E R F. Nocardiopsis synnemataformans NBRM9, an extremophilic actinomycete producing extremozyme cellulase,using lignocellulosic agro-wastes and its biotechnological applications [J]. AIMS Microbiol., 2024, 10(1):187-219. |
| 39 | WANG J, WANG Y, FENG J, et al.. Biochemical characterization of a novel endoglucanase from Thermomonospora fusca YX and its application in lignocellulosic biomass hydrolysis [J/OL]. Enzyme Microb. Technol., 2023, 167:110132 [2024-05-20]. |
| 40 | 刘晓飞,侯艳,郑志辉,等.降解玉米秸秆放线菌的筛选及发酵工艺优化[J].哈尔滨商业大学学报(自然科学版),2022,38(2):143-153. |
| LIU X F, HOU Y, ZHENG Z H, et al.. Screening of actinomycetes degrading corn stalk and optimization of fermentation process [J]. J. Harbin Univ. Commer. (Nat. Sci. ), 2022, 38(2):143-153. | |
| 41 | 曹慧,任世威,张腾月,等.青藏高原低温纤维素降解菌的筛选与酶学特性[J].饲料工业,2021,42(8):36-41. |
| CAO H, REN S W, ZHANG T Y, et al.. Screening and enzymatic properties of cold-adapted cellulose degradation bacteria in the Qinghai-Tibet Plateau [J]. Feed. Ind., 2021, 42(8):36-41. | |
| 42 | 申术霞,孟庆伟,刘金玺,等.高产蛋白酶和纤维素酶放线菌的筛选、鉴定及酶活测定[J].新农业,2022(7):6-7. |
| 43 | 邵娜娜,轩换玲,罗锋,等.一株产低温纤维素酶放线菌的分离与产酶研究[J].食品工业科技,2015,36(24):159-163, 168. |
| SHAO N N, XUAN H L, LUO F, et al.. Isolation,identification and characterization of a cold-adapted cellulase-producing psychrotrophic Streptomyces sp [J]. Sci. Technol. Food Ind., 2015, 36(24):159-163, 168. | |
| 44 | 李婷,王玥,刘中珊,等.一株降解纤维素的低温放线菌Streptomyces azureus及产酶条件优化[J].中国农学通报,2021,37(32):25-33. |
| LI T, WANG Y, LIU Z S, et al.. A novel low temperature cellulose-degrading strain Streptomyces azureus and its enzymatic production condition optimization [J]. Chin. Agric. Sci. Bull., 2021, 37(32):25-33. | |
| 45 | BERISIO R, BARRA G, ROMANO M, et al.. Structural and biochemical characterization of endo-β- 1, 4-glucanase from Dictyoglomus thermophilum, a hyperthermostable and halotolerant cellulase [J/OL]. Catalysts, 2022, 12(3): 302 [2024-05-20].. |
| 46 | RAJ T, CHANDRASEKHAR K, NARESH KUMAR A,et al.. Recent advances in commercial biorefineries for lignocellulosic ethanol production:current status,challenges and future perspectives [J/OL]. Bioresour. Technol., 2022, 344(Pt B): 126292 [2024-05-20]. . |
| 47 | 姚光山.草酸青霉纤维素合成酶合成调控机制研究及高产菌株构建[D].济南:山东大学,2016. |
| YAO G S. Studiesofthetranscription regulatory mechanism of lignocellulose-degrading enzyme synthesis and construction of cellulase high-producer in Penicillium oxalicum [D]. Jinan: Shandong University, 2016. | |
| 48 | 唐自钟,刘姗,韩学易,等.产外切葡聚糖酶真菌的筛选鉴定及毕赤酵母中的表达[J].微生物学通报,2014,41(4):629-635. |
| TANG Z Z, LIU S, HAN X Y,et al.. Screening and identification of exo-1, 4-β-D-glucannase-producing fungi, and its expression in Pichia pastoris [J]. Microbiol. China, 2014, 41(4):629-635. | |
| 49 | 郎立新.地衣芽孢杆菌GH48家族外切纤维素酶CelA的异源表达和功能研究[D].长春:东北师范大学,2019. |
| LANG L X. Heterologous expression and functional study of GH48 family exocellulase CelA from Bacillus licheniformis [D]. Changchun: Northeast Normal University, 2019. | |
| 50 | 赵楠.短小芽孢杆菌外切葡聚糖酶基因的克隆及其在毕赤酵母中的表达[D].呼和浩特:内蒙古大学,2015. |
| ZHAO N. Cloning of exocellobiohydrolase gene form Bacillus pumilus and expression in Pichia methanolica [D]. Hohhot: Inner Mongolia University, 2015. | |
| 51 | 杨志芹,高金鹏,郭秀娜,等.嗜热毁丝菌外切葡聚糖苷酶CBH1环状结构对酶活性及热稳定性的影响[J].菌物研究,2018,16(2):95-101. |
| YANG Z Q, GAO J P, GUO X N, et al.. Effects of loop structure on activity and thermostability of cellobiohydrolase 1 from Myceliophthora thermophila [J]. J. Fungal Res., 2018, 16(2):95-101. | |
| 52 | LI H M, WEN J P, ZHANG Z Y. Characterization and expression analysis of endoglucanase genes in a newly isolated Bacillus subtilis strain for enhanced cellulose degradation [J]. Biotechnol. Bioeng., 2023, 120(3): 456-467. |
| 53 | 乔宇,毛爱军,何永志,等.里氏木霉内切-β-葡聚糖酶Ⅱ基因在毕赤酵母中的表达及酶学性质研究[J].菌物学报,2004,23(3):388-396. |
| QIAO Y, MAO A J, HE Y Z, et al.. Secreted expression of Trichoderma reesei endo-β-glucanase ⅱ gene in Pichia pastoris and anylysis of enzymic properties [J]. Mycosystema, 2004, 23(3):388-396. | |
| 54 | 闫建英,张智,冯丽荣,等.绿色木霉内切葡聚糖酶基因Eg Ⅶ的克隆及其表达[J].中南林业科技大学学报,2022,42(7):169-177. |
| YAN J Y, ZHANG Z, FENG L R, et al.. Cloning and expression of Trichoderma viride Eg Ⅶ [J]. J. Central South Univ. For. Technol., 2022, 42(7): 169-177. | |
| 55 | 赵红晓.绿色木霉内切葡聚糖酶Ⅶ基因的克隆与表达[D].哈尔滨:东北农业大学,2018. |
| ZHAO H X. Cloning and expression of endoglucanase Ⅶ gene in Pichia pastoris from Trichoderma viride [D]. Harbin: Northeast Agricultural University, 2018. | |
| 56 | PANG J, WANG J S, LIU Z Y, et al.. Identification and characterization of an Endo-glucanase secreted from cellulolytic Escherichia coli ZH-4 [J/OL]. BMC Biotechnol., 2019, 19(1):63 [2024-05-20]. . |
| 57 | 佟新新,金鹏,李伟国,等.黑曲霉内切葡聚糖酶系的基本酶学特征解析[J].天津科技大学学报,2018,33(5):25-32. |
| TONG X X, JIN P, LI W G, et al.. Biochemical characterization of endoglucanases from Aspergillus niger [J]. J. Tianjin Univ. Sci. Technol., 2018, 33(5):25-32. | |
| 58 | 韩冰.黑曲霉高耐热葡聚糖酶基因克隆及其在毕赤酵母中高表达方法探索[D].无锡:江南大学,2018. |
| HAN B. Gene cloning and characterization of a higly thermal stable glucanase from Aspergillus niger and its high level expression in Pichia pastoris [D]. Wuxi: Jiangnan University, 2018. | |
| 59 | 邹爱爱,魏亚琴,杨宇泽,等.牛瘤胃纤维素酶eg基因在乳酸菌中的克隆表达及酶学性质分析[J].草业科学,2021,38(12):2471-2480. |
| ZOU A A, WEI Y Q, YANG Y Z, et al.. Expression of the eg gene of cellulase from cattle rumen in Lactobacillus and analysis of enzymatic properties [J]. Pratac. Sci., 2021, 38(12):2471-2480. | |
| 60 | VÁZQUEZ-ORTEGA P G, LÓPEZ-MIRANDA J, ROJAS-CONTRERAS J A, et al.. Expression of a β-glucosidase from Trichoderma reesei in Escherichia coli using a synthetic optimized gene and stability improvements by immobilization using magnetite nano-support [J/OL]. Protein Expr. Purif., 2022, 190: 106009 [2024-05-20]. . |
| 61 | 赵素雅,刘苏瑶,魏旭阳,等.白蚁肠道具耐高温耐碱β-葡萄糖苷酶活性共生菌株的筛选鉴定及其产酶条件优化[J].西南师范大学学报(自然科学版),2021,46(6):65-74. |
| ZHAO S Y, LIU S Y, WEI X Y, et al.. Screening and identification of bacteria from termite producing β-glucosidase and optimization of conditions for enzyme production [J]. J. Southwest China Norm. Univ. (Nat. Sci.), 2021, 46(6):65-74. | |
| 62 | 王后福,廖奇,李鹏飞,等.一株纤维降解菌β-葡萄糖苷酶基因的克隆与表达及酶学性质分析[J].饲料研究,2020,43(2):50-55. |
| WANG H F, LIAO Q, LI P F, et al.. Cloning, expression and enzymatic properties analysis of β-glucosidase gene from a fiber-degrading bacteria [J]. Feed Res., 2020, 43(2): 50-55. | |
| 63 | SUTAONEY P, RAI S N, SINHA S, et al.. Current perspective in research and industrial applications of microbial cellulases [J/OL]. Int. J. Biol.Macromol., 2024, 264(Pt 1): 130639 [2024-05-20]. . |
| 64 | ARTZI L, MORAG E, BARAK Y, et al.. Clostridium clariflavum:key cellulosome players are revealed by proteomic analysis [J/OL]. MBio, 2015, 6(3):e00411-15 [2024-05-20]. . |
| 65 | 白挨玺. 细菌Caldicellulosiruptor bescii嗜热纤维素酶系功能及协同作用研究[D]. 长春: 吉林大学, 2012. |
| BAI Y X. Functional and synergistic studies of thermophilic cellulases from Caldicellulosiruptor bescii [D]. Changchun: Jilin University, 2012. | |
| 66 | SINGH A, BAJAR S, DEVI A, et al.. An overview on the recent developments in fungal cellulase production and their industrial applications [J/OL]. Bioresour. Technol. Rep., 2021, 14:100652 [2024-05-20]. . |
| 67 | QI J Y, LI F F, JIA L, et al. .Fungal selectivity and biodegradation effects by white and brown rot fungi for wood biomass pretreatment [J/OL]. Polymers, 2023, 15(8):1957 [2024-05-20]. . |
| 68 | REESE E T, SIU R G H, LEVINSON H S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis [J]. J. Bacteriol., 1950, 59(4):485-497. |
| 69 | THAPA S, MISHRA J, ARORA N, et al.. Microbial cellulolytic enzymes: diversity and biotechnology with reference to lignocellulosic biomass degradation [J]. Rev.Environ. Sci. Bio. Technol., 2020, 19(3):621-648. |
| 70 | PLOUHINEC L, NEUGNOT V, LAFOND M, et al.. Carbohydrate-active enzymes in animal feed [J/OL]. Biotechnol.Adv., 2023, 65:108145 [2024-05-20]. . |
| 71 | JAMES A, PANDYA S, SUTAONEY P, et al.. Isolation and screening of potent cellulolytic soil fungi from raipur city of Chhattisgarh State, India [J]. J. Sci. Ind. Res., 2022, 81(11): 1173-1180. |
| 72 | BALLA A, SILINI A, CHERIF-SILINI H, et al.. Screening of cellulolytic bacteria from various ecosystems and their cellulases production under multi-stress conditions [J]. Catalysts, 2022, 12(7): 769-775. |
| 73 | FRANCO CAIRO J P L, CANNELLA D, OLIVEIRA L C, et al..On the roles of AA15 lytic polysaccharide monooxygenases derived from the termite Coptotermes gestroi [J/OL]. J. Inorg.Biochem., 2021, 216:111316 [2024-05-20]. . |
| 74 | KHAMASSI A, DUMON C. Enzyme synergy for plant cell wall polysaccharide degradation [J]. Essays Biochem., 2023, 67(3):521-531. |
| 75 | RYTIOJA J, HILDÉN K, YUZON J, et al.. Plant-polysaccharide-degrading enzymes from basidiomycetes [J]. Microbiol. Mol. Biol. Rev., 2014, 78(4):614-649. |
| 76 | NERO G, KIVIRAND K, BENS OTHMAN, et al.. Amperometric method for the determination of cellulase activity and its optimization using response surface method [J/OL]. J. Anal. Sci. Technol., 2022, 13(1):21 [2024-05-20]. . |
| 77 | PAJAK A, LEZYK B, LEDAKOWICZ S. The effect of surfactants on the activity and stability of cellulase from Trichoderma reesei using carboxymethyl cellulose as substrate [J]. Bioproc. Biosyst. Eng., 2017, 40(12): 1749-1758. |
| 78 | KOUBOVÁ A, LORENC F, HORVÁTHOVÁ T, et al.. Millipede gut-derived microbes as a potential source of cellulolytic enzymes [J/OL]. World J. Microbiol.Biotechnol., 2023, 39(7):169 [2024-05-20]. . |
| 79 | PATEL A K, SINGHANIA R R, SIM S J, et al.. Thermostable cellulases: Current status and perspectives [J]. Bioresour. Technol., 2019, 279:385-392. |
| 80 | ADNAN M, MA X K, OLSSON S, et al.. Promoter regulation and genetic engineering strategies for enhanced cellulase expression in Trichoderma reesei [J/OL]. Microbiol. Res., 2022, 259:127011 [2024-05-20]. . |
| 81 | JEENNOR S, ANANTAYANON J, CHUTRAKUL C, et al.. Novel pentose-regulated promoter of Aspergillus oryzae with application in controlling heterologous gene expression [J/OL]. Biotechnol. Rep. (amst), 2022, 33:e00695 [2024-05-20]. . |
| 82 | BALDWIN E L, KARKI B, JOHNSON T J, et al.. Enhancing cellulase production in Aureobasidium pullulans by genome shufing [J]. Indust. Biotechnol., 2020, 16(4): 247-255. |
| 83 | SUN X H, ZHANG X H, HUANG H Q, et al.. Engineering the cbh1 promoter of Trichoderma reesei for enhanced protein production by replacing the binding sites of a transcription repressor ACE1 to those of the activators [J]. J. Agric. Food Chem., 2020, 68(5):1337-1346. |
| 84 | WANG L, XIE Y J, CHANG J J, et al.. A novel sucrose-inducible expression system and its application for production of biomass-degrading enzymes in Aspergillus niger [J/OL]. Biotechnol. Biofuels Bioprod., 2023, 16(1):23 [2024-05-20]. . |
| 85 | LI X, GENG X Y, GAO L, et al.. Optimized expression of a hyperthermostable endoglucanase from Pyrococcus horikoshii in Arabidopsis thaliana [J]. Bioresources, 2019, 14(2): 2812-2826. |
| 86 | SHEN L J, YAN A Q, WANG Y F, et al..Tailoring the expression of Xyr1 leads to efficient production of lignocellulolytic enzymes in Trichoderma reesei for improved saccharification of corncob residues [J/OL]. Biotechnol.Biofuels Bioprod., 2022, 15(1):142 [2024-05-20]. . |
| 87 | DRUZHININA I S, KUBICEK C P. Genetic engineering of Trichoderma reesei cellulases and their production [J]. Microb.Biotechnol., 2017, 10(6):1485-1499. |
| 88 | YANG R F, WANG Z X, XIA Y X, et al.. Role of the nitrogen metabolism regulator TAM1 in regulation of cellulase gene expression in Trichoderma reesei [J/OL]. Appl. Environ. Microbiol., 2023, 89(1):e0142122 [2024-05-20]. . |
| 89 | WANG Q, ZHONG C, XIAO H. Genetic engineering of filamentous fungi for efficient protein expression and secretion [J/OL]. Front. Bioeng. Biotechnol., 2020, 8:293 [2024-05-20]. . |
| 90 | CHUNG D, SARAI N S, KNOTT B C, et al.. Glycosylation is vital for industrial performance of hyperactive cellulases [J]. ACS Sustainable Chem. Eng., 2019, 7(5):4792-4800. |
| 91 | WEI W, CHEN L, ZOU G, et al.. N-glycosylation affects the proper folding, enzymatic characteristics and production of a fungal β-glucosidase [J]. Biotechnol. Bioeng., 2013, 110(12):3075-3084. |
| 92 | DONG Y T, MIAO R Y, FENG R C, et al.. Edible and medicinal fungi breeding techniques,a review:current status and future prospects [J]. Curr. Res. Food Sci., 2022, 5: 2070-2080. |
| 93 | ADEBAMI G E, ADEBAYO-TAYO B C. Development of cellulolytic strain by genetic engineering approach for enhanced cellulase production [J/OL]. Genetic Metabolic Eng. Improved Biofuel Production Lignocellulosic Biomass, 2020, 8:7 [2024-05-20]. . |
| 94 | ULLAH M, XIA L, XIE S X, et al.. CRISPR/Cas9-based genome engineering:a new breakthrough in the genetic manipulation of filamentous fungi [J]. Biotechnol. Appl.Biochem., 2020, 67(6):835-851. |
| 95 | BAI S K, HONG Y, WU Y R. Emerging technologies for genetic modification of solventogenic clostridia:from tool to strategy development [J/OL]. Bioresour. Technol., 2021, 334:125222 [2024-05-20]. . |
| 96 | NØDVIG C S, NIELSEN J B, KOGLE M E, et al.. A CRISPR-Cas9 system for genetic engineering of filamentous fungi [J/OL]. PLoS One, 2015, 10(7):e0133085 [2024-05-20].. |
| 97 | LIU Q, GAO R R, LI J G, et al.. Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering [J/OL]. Biotechnol. Biofuels, 2017, 10(1):1 [2024-05-20]. . |
| 98 | MONDAL S, HALDER S K, MONDAL K C. Tailoring in fungi for next generation cellulase production with special reference to CRISPR/CAS system [J]. Syst. Microbiol. Biomanuf., 2022, 2(1):113-129. |
| 99 | 陈思彤.纤维素酶在水产养殖中的应用研究[J].饲料研究,2022,45(6):135-138. |
| CHEN S T. Research on the application of cellulase in aquaculture [J]. Feed. Res., 2022, 45(6):135-138. | |
| 100 | DE SOUZA T S P, KAWAGUTI H Y. Cellulases,hemicellulases,and pectinases:applications in the food and beverage industry [J]. Food Bioprocess Technol., 2021, 14(8):1446-1477. |
| 101 | 朱秀清,曾剑华,房媛媛,等.纤维素酶结合碱性蛋白酶提高冷榨大豆出油率的工艺优化[J].中国油脂,2019,44(5):13-17. |
| ZHU X Q, ZENG J H, FANG Y Y, et al.. Optimization of process of cellulase and alkaline protease for improving soybean oil yield by cold-pressing [J]. China Oils Fats, 2019, 44(5):13-17. | |
| 102 | 仝佳音,杨毅坚,杨方慧,等.添加多酚氧化酶和纤维素酶对大叶种红茶的品质影响[J].陕西农业科学,2020,66(5):50-53. |
| 103 | YANG G, ZHAO Q, HE B, et al.. An endocellulase-triggered NO targeted-release enzyme-prodrug therapy system and its application in ischemia injury [J/OL]. Adv. Healthc. Mater., 2024, 13(29): 2401599 [2024-05-20]. . |
| 104 | LIU Q, MADADI M, Al AZAD S, et al.. In-depth recognition of mixed surfactants maintaining the enzymatic activity of cellulases through stabilization of their spatial structures [J/OL]. Bioresour. Technol., 2025, 416: 131756 [2024-05-20]. . |
| 105 | STANESCU M D. Applications of enzymes in processing cellulosic textiles—a review of the latest developments [J]. Cellul. Chem. Technol., 2023, 57: 1-15. |
| 106 | PERUMAL A B, NAMBIAR R B, MOSES J A, et al.. Nanocellulose: recent trends and applications in the food industry [J/OL]. Food Hydrocolloids, 2022, 5(127): 107484 [2024-05-20]. . |
| 107 | DAHIYA D, KOITTO T, KUTVONEN K, et al.. Fungal loosenin-like proteins boost the cellulolytic enzyme conversion of pretreated wood fiber and cellulosic pulps [J]. Bioresour. Technol., 2024, 394: 130188 [2024-05-20]. . |
| 108 | 卜杰.嗜热厌氧纤维素降解产氢菌的选育以及生物炭强化厌氧发酵性能的机理研究[D].广州:华南理工大学,2022. |
| BU J. The breeding of Thermophilic anaerobic cellulolytic and hydrogen-producing strains and study on the enhanced mechanisms of anaerobic fermentative performance by biochar amendment [D]. Guangzhou: South China University of Technology, 2022. |
| [1] | 蓝江林, 肖荣凤, 王阶平, 张海峰, 刘波. 整合微生物组菌剂对番茄植株生长及根际细菌群落多样性的影响[J]. 中国农业科技导报, 2025, 27(5): 173-181. |
| [2] | 吴小丹, 高丽, 巩天耕, 孔祥凤, 姜雨舟, 贾桂霞. 微生物菌肥和腐植酸复合肥对百合生长和光合特性的影响[J]. 中国农业科技导报, 2025, 27(4): 221-229. |
| [3] | 邵鹏阳, 沙玉柱, 刘秀, 陈国顺, 朱才业, 王继卿, 王翻兄, 陈小伟, 杨文鑫. 黄芪饲料添加剂对羔羊生长性能、血清Ig和瘤胃发酵功能及微生物菌群特征的影响[J]. 中国农业科技导报, 2025, 27(3): 83-94. |
| [4] | 王跃锋, 张晨阳, 罗正明, 李建华, 李然, 孙楠, 徐明岗. 整合分析添加有机物料对我国农田土壤微生物残体的影响[J]. 中国农业科技导报, 2025, 27(2): 180-191. |
| [5] | 马振华, 时倩茹, 宁欣杰, 魏宏杨, 王璨, 张静静, 张彪, 杨素勤. 生物质炭对镉铅污染土壤线虫群落的影响[J]. 中国农业科技导报, 2025, 27(2): 201-210. |
| [6] | 熊橙梁, 张庆富, 姚未远, 夏滔, 许庆平, 周喜新, 张毅, 陈丽鹃, 杨柳. 添加不同类型水稻秸秆对植烟连作土壤微生物群落的影响[J]. 中国农业科技导报, 2025, 27(1): 233-240. |
| [7] | 王吉平, 卢铁东, 梁芷姮, 张野, 苏天明, 何铁光. 不同来源微生物对葡萄枝条猪粪共堆肥过程的影响[J]. 中国农业科技导报, 2024, 26(9): 224-233. |
| [8] | 王兴松, 王娜, 杜宇, 周鹏, 王戈, 贾孟, 徐照丽, 白羽祥. 有机肥对玉溪植烟土壤有机质组分和微生物群落结构的影响[J]. 中国农业科技导报, 2024, 26(8): 201-212. |
| [9] | 张继东, 张亚雄, 程伟, 蒲莉, 柳路行, 王亚明. 生物质炭和有机肥配施对苹果重茬育苗地土壤理化性质和微生物群落特征的影响[J]. 中国农业科技导报, 2024, 26(8): 213-222. |
| [10] | 姜坤宏, 许祯莹, 郭真真, 白林, 郝晓霞, 姜冬梅, 邱时秀. 微生物电化学技术原理及其在畜禽废弃物资源化领域的应用研究进展[J]. 中国农业科技导报, 2024, 26(7): 210-222. |
| [11] | 王子凡, 李燕, 张庆银, 王丹丹, 师建华, 耿晓彬, 田东良, 钟增明, 赵晓明, 齐连芬. 微生物菌剂对设施番茄主要病害及土壤微生物群落的影响[J]. 中国农业科技导报, 2024, 26(6): 102-112. |
| [12] | 强敬雯, 王晚晴, 唐曼玉, 张娜, 武双, 华威, 邵恒煊, 程艳玲. 厨余垃圾厌氧消化对沼气微生物及环境的影响[J]. 中国农业科技导报, 2024, 26(6): 159-169. |
| [13] | 赵鸿硕, 曹红雨, 高广磊, 孙哲, 张英, 丁国栋. 微生物诱导碳酸钙沉淀固沙对典型沙生植物叶片性状和生理特性的影响[J]. 中国农业科技导报, 2024, 26(6): 170-182. |
| [14] | 陈雨欣, 赵红梅, 杨卫君, 杨梅, 郭颂, 宋世龙, 惠超. 生物质炭对土壤微生物碳源利用及春小麦产量的影响[J]. 中国农业科技导报, 2024, 26(5): 174-183. |
| [15] | 杜洋洋, 包媛媛, 刘项宇, 张新永. 荞麦轮作对云南栽培马铃薯根际土壤酶活和微生物的影响[J]. 中国农业科技导报, 2024, 26(5): 192-200. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号