




















中国农业科技导报 ›› 2022, Vol. 24 ›› Issue (6): 58-69.DOI: 10.13304/j.nykjdb.2021.0692
王莉莉1,5( ), 殷丛培2(
), 殷丛培2( ), 李峰3, 杨志敏3, 刘芳明1, 林柏松1, 刘晓静1, 刘海军4, 孙靖4, 单东东4, 崔江慧2,5(
), 李峰3, 杨志敏3, 刘芳明1, 林柏松1, 刘晓静1, 刘海军4, 孙靖4, 单东东4, 崔江慧2,5( ), 张振清4,5(
), 张振清4,5( )
)
收稿日期:2021-08-12
接受日期:2021-11-15
出版日期:2022-06-15
发布日期:2022-06-21
通讯作者:
崔江慧,张振清
作者简介:王莉莉 E-mail:546156736@qq.com基金资助:
Lili WANG1,5( ), Congpei YIN2(
), Congpei YIN2( ), Feng LI3, Zhimin YANG3, Fangming LIU1, Baisong LIN1, Xiaojing LIU1, Haijun LIU4, Jing SUN4, Dongdong SHAN4, Jianghui CUI2,5(
), Feng LI3, Zhimin YANG3, Fangming LIU1, Baisong LIN1, Xiaojing LIU1, Haijun LIU4, Jing SUN4, Dongdong SHAN4, Jianghui CUI2,5( ), Zhenqing ZHANG4,5(
), Zhenqing ZHANG4,5( )
)
Received:2021-08-12
Accepted:2021-11-15
Online:2022-06-15
Published:2022-06-21
Contact:
Jianghui CUI,Zhenqing ZHANG
摘要:
为研究马铃薯根际土壤细菌的群落结构及其对干旱胁迫的响应,采用盆栽试验,以开花期马铃薯根际土壤为研究对象,提取其总DNA,通过Illumina Miseq对细菌 V3~V4区进行高通量测序,对干旱处理下马铃薯根际微生物的群落结构进行分析。结果表明,共鉴定有效序列1 263 889条,6 785个OTUs归属33个门、94个纲、267个目、462个科、919个属、1 930个种。与对照土壤相比,种植马铃薯后根际土壤中的变形菌门(Proteobacteria)、放线菌门(Actinobacteria)、酸杆菌门(Acidobacteria)、绿弯菌门(Chloroflexi)、拟杆菌门(Bacteroidetes)、厚壁菌门(Firmicutes)和芽单胞菌门(Gemmatimonadetes)为优势菌门,其中放线菌门(Proteobacteria)的相对丰度显著增加。干旱处理提高了马铃薯根际土壤中变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、芽单胞菌门(Gemmatimonadetes)和假单胞菌属(Pseudomonas)的相对丰度。PCoA(principal coordinates analysis)分析显示,种植马铃薯和干旱胁迫是造成土壤细菌群落结构变异的重要因素。PICRUSt(phylogenetic investigation of communities by reconstruction of unobserved states)分析表明,干旱处理后根际土壤细菌的变化与马铃薯植株的生理代谢、信号转导、防御机制和基本生命活性等密切相关,对植物的生存和耐旱性具有重要意义,为通过改良土壤微生物环境来提高植物胁迫耐受性提供了重要参考。
中图分类号:
王莉莉, 殷丛培, 李峰, 杨志敏, 刘芳明, 林柏松, 刘晓静, 刘海军, 孙靖, 单东东, 崔江慧, 张振清. 马铃薯根际土壤细菌群落结构及其对干旱胁迫的响应[J]. 中国农业科技导报, 2022, 24(6): 58-69.
Lili WANG, Congpei YIN, Feng LI, Zhimin YANG, Fangming LIU, Baisong LIN, Xiaojing LIU, Haijun LIU, Jing SUN, Dongdong SHAN, Jianghui CUI, Zhenqing ZHANG. Microbial Community Structure of Potato Rhizosphere Soil and Its Response to Drought Stress[J]. Journal of Agricultural Science and Technology, 2022, 24(6): 58-69.
| 品种Variety | 处理Treatment | 净光合速率Pn/(µmol·m-2·s-1) | 蒸腾速率 Tr/(mmol·m-2·s-1) | 气孔导度 Gs/(mol·m-2·s-1) | 胞间CO2浓度 Ci/(µmol·mol-1) |
|---|---|---|---|---|---|
| 冀张薯8号 Jizhangshu 8 | T0 | 22.00±2.12 a | 2.35±0.33 a | 13.83±1.10 a | 170.33±10.66 a |
| T1 | 24.80±9.04 a | 2.27±0.21 a | 10.33±0.74 b | 178.53±16.03 a | |
| T2 | 17.44±3.10 ab | 2.01±0.21 ab | 8.50±0.89 bc | 182.67±9.18 a | |
| T3 | 13.91±1.08 b | 1.79±0.05 ab | 6.00±0.47 c | 195.41±9.50 a | |
夏波蒂 Shepody | T0 | 28.01±2.22 a | 2.79±0.16 a | 13.76±1.35 a | 137.42±11.03 b |
| T1 | 18.63±3.66 a | 2.17±0.45 b | 11.67±1.06 ab | 159.00±3.65 ab | |
| T2 | 14.73±2.00 b | 1.58±0.32 b | 8.87±1.51 bc | 173.14±7.11 a | |
| T3 | 8.97±1.82 c | 1.03±0.11 c | 4.80±1.08 c | 180.00±13.12 a |
表1 不同处理下马铃薯叶片的光合特性
Table 1 Photosynthetic characteristics of potato leaves under different treatments
| 品种Variety | 处理Treatment | 净光合速率Pn/(µmol·m-2·s-1) | 蒸腾速率 Tr/(mmol·m-2·s-1) | 气孔导度 Gs/(mol·m-2·s-1) | 胞间CO2浓度 Ci/(µmol·mol-1) |
|---|---|---|---|---|---|
| 冀张薯8号 Jizhangshu 8 | T0 | 22.00±2.12 a | 2.35±0.33 a | 13.83±1.10 a | 170.33±10.66 a |
| T1 | 24.80±9.04 a | 2.27±0.21 a | 10.33±0.74 b | 178.53±16.03 a | |
| T2 | 17.44±3.10 ab | 2.01±0.21 ab | 8.50±0.89 bc | 182.67±9.18 a | |
| T3 | 13.91±1.08 b | 1.79±0.05 ab | 6.00±0.47 c | 195.41±9.50 a | |
夏波蒂 Shepody | T0 | 28.01±2.22 a | 2.79±0.16 a | 13.76±1.35 a | 137.42±11.03 b |
| T1 | 18.63±3.66 a | 2.17±0.45 b | 11.67±1.06 ab | 159.00±3.65 ab | |
| T2 | 14.73±2.00 b | 1.58±0.32 b | 8.87±1.51 bc | 173.14±7.11 a | |
| T3 | 8.97±1.82 c | 1.03±0.11 c | 4.80±1.08 c | 180.00±13.12 a |
| 品种Variety | 处理 Treatment | 超氧化物歧化酶活性 SOD activity/(U·min-1) | 过氧化物酶活性 POD activity /(U·min-1) | 过氧化氢酶活性 CAT activity /(U·min-1) |
|---|---|---|---|---|
冀张薯8号 Jizhangshu 8 | T0 | 118.04±8.77 b | 73.50±2.45 c | 5.38±0.20 b |
| T1 | 123.74±6.62 b | 93.76±4.71 b | 6.70±0.61 ab | |
| T2 | 145.98±7.02 b | 98.35±5.03 b | 8.01±0.30 a | |
| T3 | 240.01±9.22 a | 119.98±6.73 a | 5.30±0.40 b | |
夏波蒂 Shepody | T0 | 44.98±5.66 c | 74.03±2.07 c | 4.66±0.31 a |
| T1 | 58.06±4.08 b | 94.03±3.99 b | 4.76±0.58 a | |
| T2 | 67.02±5.31 ab | 97.77±3.22 b | 4.72±0.57 a | |
| T3 | 87.96±6.33 a | 118.03±4.14 a | 3.13±0.71 b |
表 2 干旱胁迫下马铃薯叶片保护酶的活性
Table 2 Enzyme activities of potato leaves under different treatments
| 品种Variety | 处理 Treatment | 超氧化物歧化酶活性 SOD activity/(U·min-1) | 过氧化物酶活性 POD activity /(U·min-1) | 过氧化氢酶活性 CAT activity /(U·min-1) |
|---|---|---|---|---|
冀张薯8号 Jizhangshu 8 | T0 | 118.04±8.77 b | 73.50±2.45 c | 5.38±0.20 b |
| T1 | 123.74±6.62 b | 93.76±4.71 b | 6.70±0.61 ab | |
| T2 | 145.98±7.02 b | 98.35±5.03 b | 8.01±0.30 a | |
| T3 | 240.01±9.22 a | 119.98±6.73 a | 5.30±0.40 b | |
夏波蒂 Shepody | T0 | 44.98±5.66 c | 74.03±2.07 c | 4.66±0.31 a |
| T1 | 58.06±4.08 b | 94.03±3.99 b | 4.76±0.58 a | |
| T2 | 67.02±5.31 ab | 97.77±3.22 b | 4.72±0.57 a | |
| T3 | 87.96±6.33 a | 118.03±4.14 a | 3.13±0.71 b |
处理 Treatment | 有效序列数 Sequence number | 碱基数 Base number | 平均长度 Mean length/bp | 最短序列长度 Min. length/bp | 最长序列长度 Max. length/bp |
|---|---|---|---|---|---|
| 对照土CK soil | 173 462 | 72 743 138 | 419.36 | 257 | 480 |
| S-T0 | 150 075 | 62 852 665 | 418.84 | 276 | 472 |
| S-T1 | 125 549 | 52 301 567 | 416.60 | 289 | 455 |
| S-T2 | 159 305 | 66 521 294 | 417.57 | 243 | 476 |
| S-T3 | 178 086 | 74 359 015 | 417.53 | 271 | 471 |
| JZS-T0 | 171 403 | 71 496 233 | 417.13 | 214 | 468 |
| JZS-T1 | 157 105 | 65 548 212 | 417.20 | 271 | 474 |
| JZS-T2 | 161 144 | 67 378 509 | 418.09 | 240 | 504 |
| JZS-T3 | 161 222 | 67 470 566 | 418.48 | 235 | 490 |
表3 不同处理下根际细菌群落的测序质量
Table 3 Sequencing quantity of rhizosphere bacteria under different treatments
处理 Treatment | 有效序列数 Sequence number | 碱基数 Base number | 平均长度 Mean length/bp | 最短序列长度 Min. length/bp | 最长序列长度 Max. length/bp |
|---|---|---|---|---|---|
| 对照土CK soil | 173 462 | 72 743 138 | 419.36 | 257 | 480 |
| S-T0 | 150 075 | 62 852 665 | 418.84 | 276 | 472 |
| S-T1 | 125 549 | 52 301 567 | 416.60 | 289 | 455 |
| S-T2 | 159 305 | 66 521 294 | 417.57 | 243 | 476 |
| S-T3 | 178 086 | 74 359 015 | 417.53 | 271 | 471 |
| JZS-T0 | 171 403 | 71 496 233 | 417.13 | 214 | 468 |
| JZS-T1 | 157 105 | 65 548 212 | 417.20 | 271 | 474 |
| JZS-T2 | 161 144 | 67 378 509 | 418.09 | 240 | 504 |
| JZS-T3 | 161 222 | 67 470 566 | 418.48 | 235 | 490 |
处理 Treatment | 测序深度 Sequencing depth coverage | 丰度指数 Richness index | 多样性指数 Diversity index | 均匀度指数 Evenness index | ||||
|---|---|---|---|---|---|---|---|---|
| Sobs | Chao | ACE | Shannon | Simpson | Shannon | Simpson | ||
| 对照土CK soil | 0.956 3 | 2 871.00±46.89 a | 4 019.46±124.69 a | 4 226.19±454.79 a | 6.30±0.04 e | 0.007±0.001 b | 0.791±0.003 e | 0.049±0.005 cd |
| S-T0 | 0.963 4 | 3 034.00±126.57 a | 4 300.03±193.64 a | 4 285.13±178.17 a | 6.64±0.04 b | 0.004±0.001 bcd | 0.828±0.007 b | 0.076±0.009 b |
| S-T1 | 0.962 7 | 2 914.33±332.18 a | 4 168.09±393.31 a | 4 306.80±129.02 a | 6.75±0.03 a | 0.003±0.000 d | 0.846±0.008 a | 0.117±0.016 a |
| S-T2 | 0.969 5 | 2 891.67±143.07 a | 4 154.81±341.77 a | 4 412.31±699.81 a | 6.49±0.04 cd | 0.004±0.000 bcd | 0.813±0.001 bcd | 0.078±0.002 b |
| S-T3 | 0.972 9 | 3 093.67±146.73 a | 4 318.33±190.86 a | 4 331.86±175.96 a | 6.53±0.02 cd | 0.005±0.000 bcd | 0.813±0.002 cd | 0.066±0.001 bc |
| JZS-T0 | 0.967 6 | 3 123.00±187.28 a | 4 409.18±296.88 a | 4 391.73±254.61 a | 6.46±0.12 d | 0.010±0.004 a | 0.802±0.015 de | 0.036±0.018 d |
| JZS-T1 | 0.970 9 | 2 999.00±184.52 a | 4 260.66±277.70 a | 4 209.69±243.27 a | 6.58±0.03 bc | 0.004±0.000 cd | 0.822±0.003 bc | 0.085±0.010 b |
| JZS-T2 | 0.970 0 | 3 011.67±127.44 a | 4 273.25±239.49 a | 4 240.67±228.24 a | 6.52±0.06 cd | 0.005±0.001 bcd | 0.814±0.009 bcd | 0.075±0.015 b |
| JZS-T3 | 0.969 6 | 3 015.67±100.95 a | 4 256.61±110.02 a | 4 253.03±136.65 a | 6.45±0.07 d | 0.006±0.001 bc | 0.804±0.010 de | 0.054±0.009 cd |
表4 各处理根际土壤样本 Alpha 多样性指数
Table 4 Alpha diversity index of rhizosphere soil samples in each treatment
处理 Treatment | 测序深度 Sequencing depth coverage | 丰度指数 Richness index | 多样性指数 Diversity index | 均匀度指数 Evenness index | ||||
|---|---|---|---|---|---|---|---|---|
| Sobs | Chao | ACE | Shannon | Simpson | Shannon | Simpson | ||
| 对照土CK soil | 0.956 3 | 2 871.00±46.89 a | 4 019.46±124.69 a | 4 226.19±454.79 a | 6.30±0.04 e | 0.007±0.001 b | 0.791±0.003 e | 0.049±0.005 cd |
| S-T0 | 0.963 4 | 3 034.00±126.57 a | 4 300.03±193.64 a | 4 285.13±178.17 a | 6.64±0.04 b | 0.004±0.001 bcd | 0.828±0.007 b | 0.076±0.009 b |
| S-T1 | 0.962 7 | 2 914.33±332.18 a | 4 168.09±393.31 a | 4 306.80±129.02 a | 6.75±0.03 a | 0.003±0.000 d | 0.846±0.008 a | 0.117±0.016 a |
| S-T2 | 0.969 5 | 2 891.67±143.07 a | 4 154.81±341.77 a | 4 412.31±699.81 a | 6.49±0.04 cd | 0.004±0.000 bcd | 0.813±0.001 bcd | 0.078±0.002 b |
| S-T3 | 0.972 9 | 3 093.67±146.73 a | 4 318.33±190.86 a | 4 331.86±175.96 a | 6.53±0.02 cd | 0.005±0.000 bcd | 0.813±0.002 cd | 0.066±0.001 bc |
| JZS-T0 | 0.967 6 | 3 123.00±187.28 a | 4 409.18±296.88 a | 4 391.73±254.61 a | 6.46±0.12 d | 0.010±0.004 a | 0.802±0.015 de | 0.036±0.018 d |
| JZS-T1 | 0.970 9 | 2 999.00±184.52 a | 4 260.66±277.70 a | 4 209.69±243.27 a | 6.58±0.03 bc | 0.004±0.000 cd | 0.822±0.003 bc | 0.085±0.010 b |
| JZS-T2 | 0.970 0 | 3 011.67±127.44 a | 4 273.25±239.49 a | 4 240.67±228.24 a | 6.52±0.06 cd | 0.005±0.001 bcd | 0.814±0.009 bcd | 0.075±0.015 b |
| JZS-T3 | 0.969 6 | 3 015.67±100.95 a | 4 256.61±110.02 a | 4 253.03±136.65 a | 6.45±0.07 d | 0.006±0.001 bc | 0.804±0.010 de | 0.054±0.009 cd |
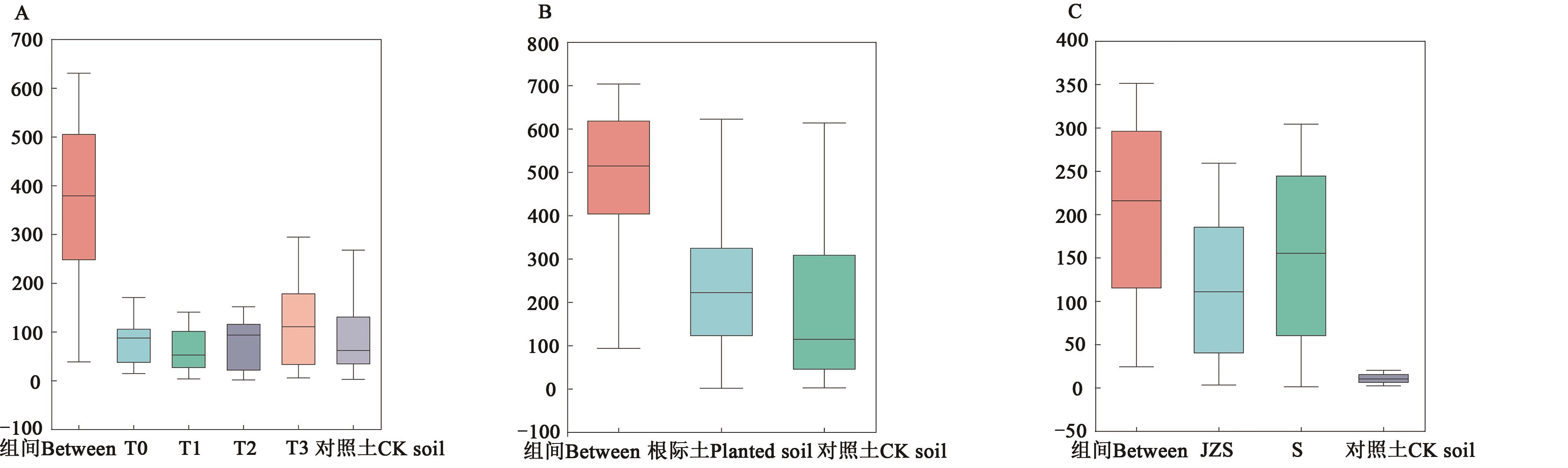
图1 3种分组方式的组内和组间差异ANOSIM分析A:品种;B:空白对照土与马铃薯根际土;C:干旱胁迫
Fig. 1 ANOSIM analysis of differences between groups and groups in 3 grouping methodsA: variety;B: CK soil and planted soil;C: drought stress
| 分组Group | R值R value | P值P value |
|---|---|---|
| A | 0.416 7 | 0.001 |
| B | 0.774 4 | 0.001 |
| C | 0.907 2 | 0.001 |
表5 相似性分析
Table 5 Analysis of similarties
| 分组Group | R值R value | P值P value |
|---|---|---|
| A | 0.416 7 | 0.001 |
| B | 0.774 4 | 0.001 |
| C | 0.907 2 | 0.001 |
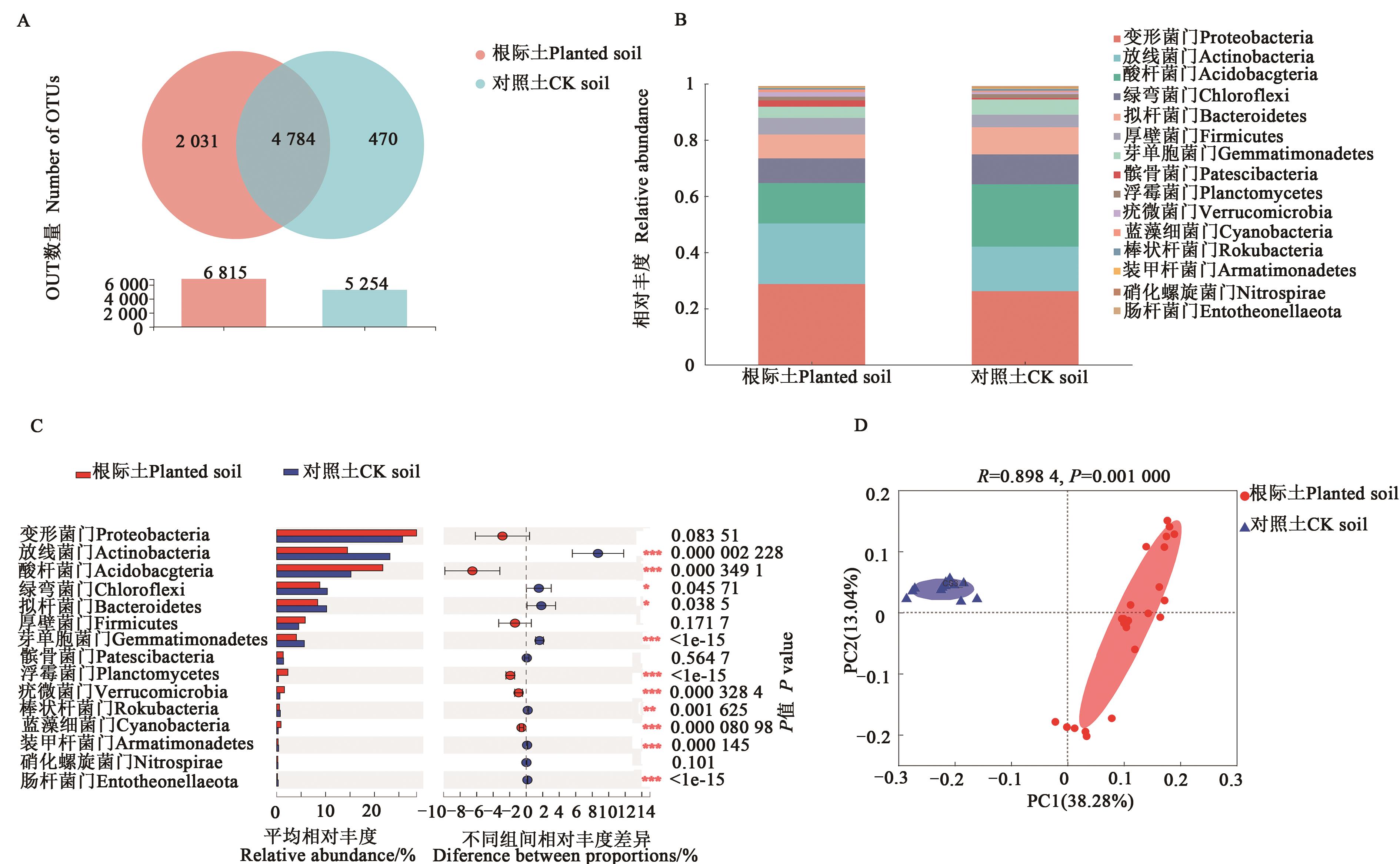
图2 种植马铃薯后土壤细菌多样性A:OTUs分析;B:门水平优势细菌的相对丰度;C:组间差异;D:PCoA分析。*、**、***分别表示在P<0.05、P<0.01和P<0.001水平差异显著。
Fig. 2 Effects of planted potato on bacterial diversityA:OTUs analysis;B:Relative abundance of dominant bacteria at phylum level;C:Differences between CK and rhizosphere soils;D:PCoA analysis.*,** and *** indicate significant differences at P<0.05,P<0.01 and P<0.001 levels,respectively.
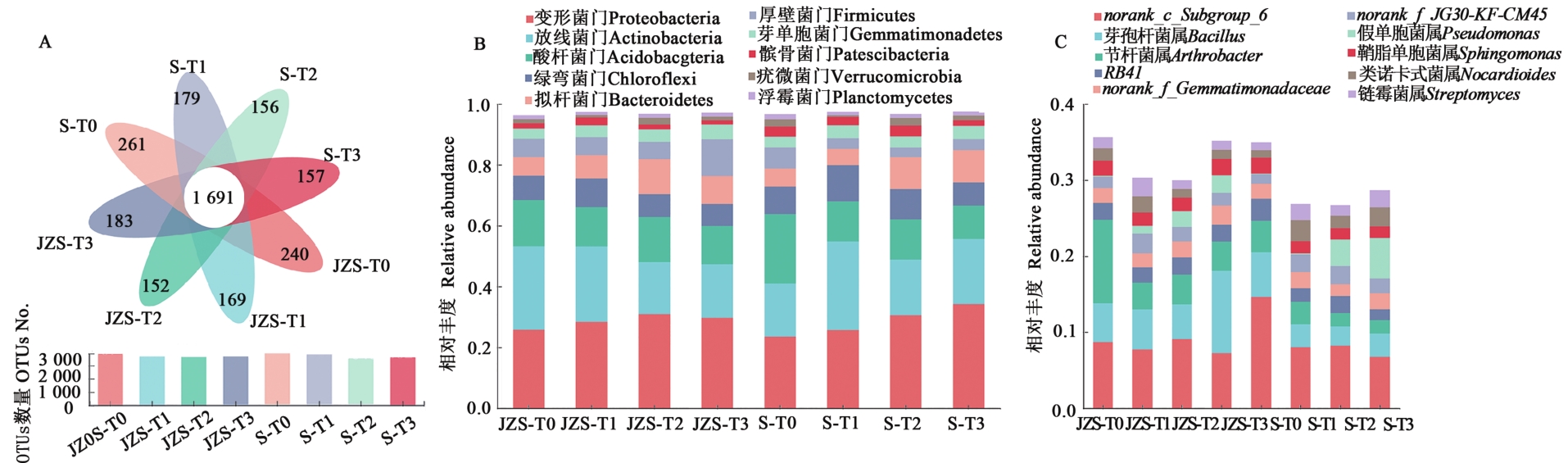
图3 干旱胁迫下马铃薯根际细菌群落结构A:OTUs 分析;B:门水平;C:属水平
Fig. 3 Bacterial community abundance in potato rhizosphere under drought stressA: OTUs analysis; B: Phylum level; C: Genus level in potato rhizosphere
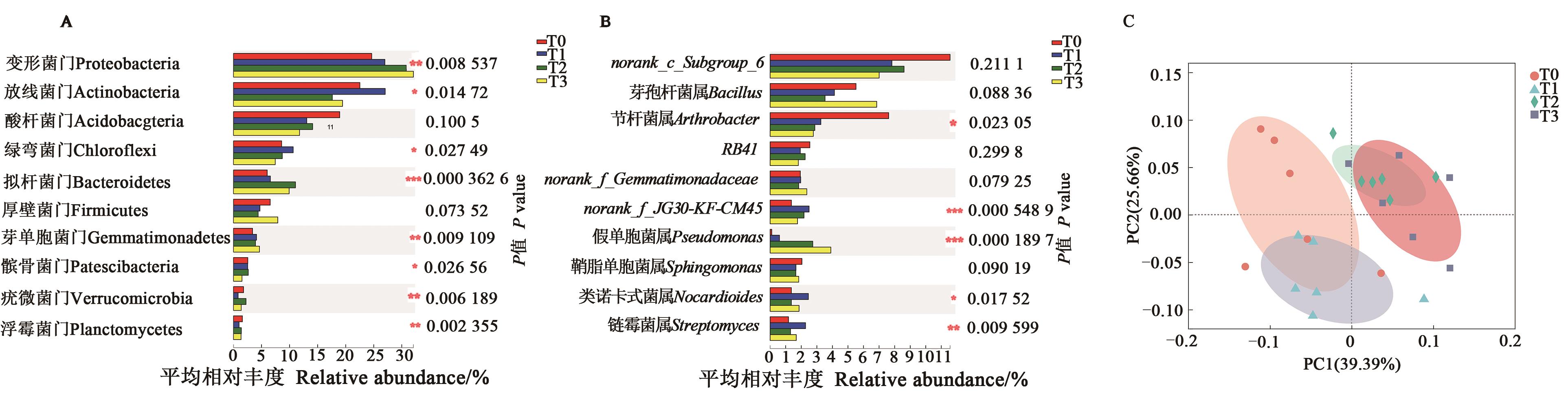
图4 不同干旱胁迫下马铃薯根际菌群组间差异注:A—门水平;B—属水平;C:门水平PCoA分析;*、**、***分别表示在P<0.05、P<0.01和P<0.001水平差异显著。
Fig. 4 Difference and distribution of rhizosphere soils under drought stressNote:A—Phylum level; B—Genus level; C—PCoA analysis on Phylum level. *, ** and *** indicate significant differences at P<0.05, P<0.01 and P<0.001 levels, respectively.
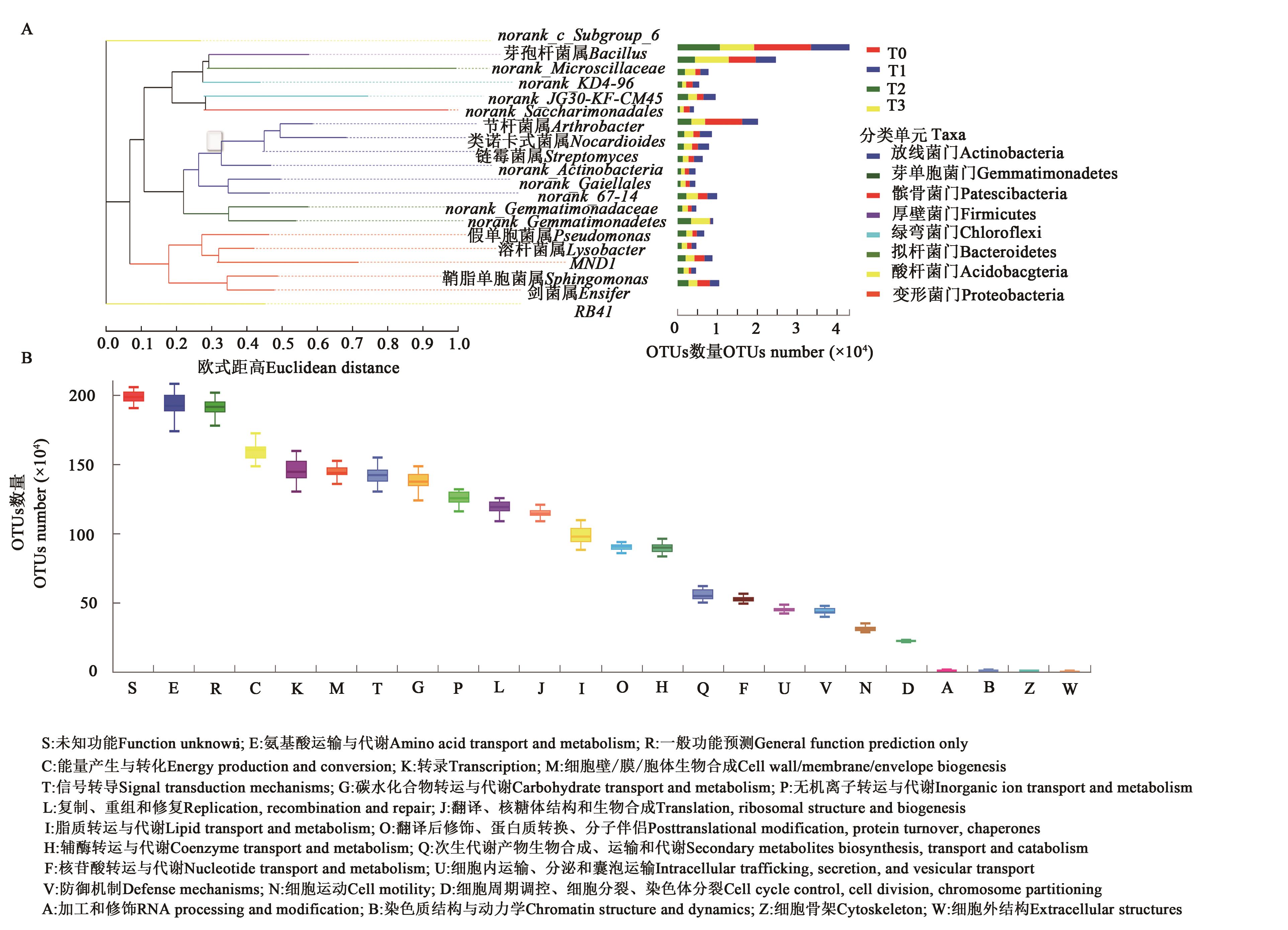
图5 根际微生物的系统发生树及功能预测A:系统进化分析;B:根际微生物的功能预测
Fig. 5 Taxonomic analysis through phylogenetic tree and microbial functional featuresA: Phylogenetic analysis; B: Functional features of rhizosphere microbe
| 1 | AKSOY E, DEMİREL U, ÖZTÜRK Z N, et al.. Recent advances in potato genomics, transcriptomics, and transgenics under drought and heat stresses: a review [J]. Turkish J. Bot., 2015, 39:920-940. |
| 2 | BOUDSOCQ M, LAURIERE C. Osmotic signaling in plants: multiple pathways mediated by emerging kinase families [J]. Plant Physiol., 2005, 138:1185-1194. |
| 3 | XU Y, ZHENG X, SONG Y, et al.. NtLTP4, a lipid transfer protein that enhances salt and drought stresses tolerance in Nicotiana tabacum [J/OL]. Sci. Rep., 2018, 8:8873 [2021-07-10]. . |
| 4 | XU L, NAYLOR D, DONG Z, et al.. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria [J]. Proc. Natl. Acad. Sci. USA, 2018, 115(18):4284-4293. |
| 5 | YUAN Q, DRUZHININA I S, PAN X, et al.. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture [J]. Biotechnol. Adv., 2016, 34(7):1245-1259. |
| 6 | ZHANG L, ZHANG J, WEI Y, et al.. Microbiome-wide association studies reveal correlations between the structure and metabolism of the rhizosphere microbiome and disease resistance in Cassava [J]. Plant Biotechnol. J., 2021, 19(4):689-701. |
| 7 | HOU S, THIERGART T, VANNIER N, et al.. A microbiota-root-shoot circuit favours Arabidopsis growth over defence under suboptimal light [J]. Nat. Plants, 2021, 7:1078-1092. |
| 8 | WAGNER M R, TANG C, SALVATO F, et al.. Microbe-dependent heterosis in maize [J/OL]. Proc. Natl. Acad. Sci. USA, 2021, 118(30): e2021965118 [2021-07-10]. . |
| 9 | EDWARDS J, JOHNSON C, SANTOS-MEDELLÍN C, et al.. Structure, variation, and assembly of the root-associated microbiomes of rice [J]. Proc. Natl. Acad. Sci. USA, 2015, 112 (8):911-920. |
| 10 | GENG L L, SHAO G X, RAYMOND B, et al.. Subterranean infestation by Holotrichia parallela larvae is associated with changes in the peanut (Arachis hypogaea L.) rhizosphere microbiome [J]. Microbiol. Res., 2018, 211:13-20. |
| 11 | BAI Y, MULLER D B, SRINIVAS G, et al.. Functional overlap of the Arabidopsis leaf and root microbiota [J]. Nature, 2015, 528(7582): 364-369. |
| 12 | SAREH R, MAJID T, BAHRAM B, et al.. The role of plant growth-promoting rhizobacteria (PGPR) in improving iron acquisition by altering physiological and molecular responses in quince seedlings [J]. Plant Physiol. Biochem., 2020, 155:406-415. |
| 13 | VAROQUAUX N, COLE B, GAO C, et al.. Transcriptomic analysis of field-droughted sorghum from seedling to maturity reveals biotic and metabolic responses [J]. Proc. Natl. Acad. Sci. USA, 2019, 116:27124-27132. |
| 14 | VRIES F T, GRIFFITHS R I, KNIGHT C G, et al.. Harnessing rhizosphere microbiomes for drought-resilient crop production [J]. Science, 2020, 368(6488):270-274. |
| 15 | XU L, DONG Z, CHINIQUY D, et al.. Genome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics [J/OL]. Nat. Commun., 2021, 12(1): 3209 [2021-07-10]. . |
| 16 | XU L, COLEMAN-DERR D. Causes and consequences of a conserved bacterial root microbiome response to drought stress [J]. Curr. Opin. Microbiol., 2019, 49:1-6. |
| 17 | 高玉坤,杨溥原,项晓冬,等.不同耐盐高粱品种全生育期对盐胁迫的响应[J].华北农学报,2020,35(6):113-121. |
| GAO Y K, YANG P Y, XIANG X D, et al.. Response of different salt tolerant sorghum varieties to salt stress in the whole growth period [J]. Acta Agric. Boreali-Sin., 2020, 35(6):113-121. | |
| 18 | XU N, TAN G, WANG H, et al.. Effect of biochar additions to soil on nitr ogen leaching, microbial biomass and bacterial community structure [J]. Eur. J. Soil Biol., 2016, 74:1-8. |
| 19 | 抗艳红,龚学臣,赵海超,等.不同生育时期干旱胁迫对马铃薯生理生化指标的影响[J].中国农学通报, 2011, 27(15): 97-101. |
| KANG Y H, GONG X C, ZHAO H C, et al.. Physiological and biochemical response of potato under the drought stress in different growth period [J]. Chin. Agric. Sci. Bull., 2011, 27(15):97-101. | |
| 20 | 黄文莉,马杰,江敏,等.干旱胁迫对马铃薯抗旱生理影响及相关基因的表达[J].分子植物育种,2021, 19(21):7213-7221. |
| HUANG W L, MA J, JIANG M, et al.. Changes in drought resistance physiology and related gene expression of potato upon drought stresses [J]. Mol. Plant Breeding, 2021, 19(21):7213-7221. | |
| 21 | 曹逼力,李炜蔷,徐坤.干旱胁迫下硅对番茄叶片光合荧光特性的影响[J].植物营养与肥料学报,2016, 22(2):495-501. |
| CAO B L, LI W Q, XU K, et al.. Effects of silicon on photosynthetic and fluorescence characteristics of tomato leaves under drought stress [J]. Plant Nutr. Fert. Sci., 2016, 22(2):495-501. | |
| 22 | 梁丽娜,刘雪,唐勋,等.干旱胁迫对马铃薯叶片生理生化指标的影响[J].基因组学与应用生物学,2018,37(3):1343-1348. |
| LIANG L N, LIU X, TANG X, et al.. Effect of drought stress on physiological and biochemical indexes of potato leaves [J]. Genom. Appl. Biol., 2018, 37(3):1343-1348. | |
| 23 | 颜朗,张义正,方志荣,等.不同马铃薯基因型对根际细菌群落结构的影响[J].四川大学学报(自然科学版),2020,57(2):383-390. |
| YAN L, ZHANG Y Z, FANG Z R, et al.. Effects of potato genotype on rhizosphere bacterial community structure [J]. J. Sichuan Univ. (Nat. Sci.), 2020, 57(2):383-390. | |
| 24 | GSCHWENDTNER S, ESPERSCHÜTZ J, BUEGGER F, et al.. Effects of genetically modified starch metabolism in potato plants on photosynthate fluxes into the rhizosphere and on microbial degraders of root exudates [J]. FEMS Microbiol. Ecol., 2011, 76:564-575. |
| 25 | BULGARELLI D, GARRIDO-OTER R, MUNCH P C, et al.. Structure and function of the bacterial root microbiota in wild and domesticated barley [J]. Cell Host Microbiol., 2015, 17:392-403. |
| 26 | DAI L, ZHANG G, YU Z, et al.. Effect of drought stress and developmental stages on microbial community structure and diversity in peanut rhizosphere soil [J/OL]. Int. J. Mol. Sci., 2019, 20(9): 2265 [2021-07-10]. . |
| 27 | LUNDBERG D S, LEBEIS S L, PAREDES S H, et al.. Defining the core Arabidopsis thaliana root microbiome [J]. Nature, 2012, 488:86-90. |
| 28 | GAO Y K, CUI J H, REN G Z, et al.. Changes in the root-associated bacteria of sorghum are driven by the combined effects of salt and sorghum development [J]. Environ. Microbiome, 2021, 16:14-24. |
| 29 | DEBRUYN J M, NIXON L T, FAWAZ M N, et al.. Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil [J]. Appl. Environ. Microbiol., 2011, 77:6295-6300. |
| 30 | FOZO E M, QUIVEY R G J. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments [J]. Appl. Environ. Microbiol., 2004, 70:929-936. |
| 31 | 孙建平,刘雅辉,左永梅,等.盐地碱蓬根际土壤细菌群落结构及其功能[J].中国生态农业学报(中英文),2020,28(10):1618-1629. |
| SUN J P, LIU Y H, ZUO Y M, et al.. The bacterial community structure and function of Suaeda salsa rhizosphere soil [J]. Chin. J. Eco-Agric., 2020, 28(10):1618-1629. | |
| 32 | VACHERON J, DESBROSSES G, BOUFFAUD M L, et al.. Plant growth-promoting rhizobacteria and root system functioning [J/OL]. Front. Plant Sci., 2013, 4: 356 [2021-07-10]. . |
| 33 | NUMAN M, BASHIR S, KHAN Y, et al.. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review [J]. Microbiol. Res., 2018, 209:21-32. |
| 34 | GUAN P, WANG J, LI H, et al.. SENSITIVE TO SALT1, an endoplasmic reticulum-localized chaperone, positively regulates salt resistance [J]. Plant Physiol., 2018, 178:1390-1405. |
| 35 | WU L, WANG J, WU H, et al.. Comparative metagenomic analysis of rhizosphere microbial community composition and functional potentials under Rehmannia glutinosa consecutive monoculture [J/OL]. Int. J. Mol. Sci., 2018, 19:2394 [2021-07-10]. . |
| 36 | 吴林坤,林向民,林文雄.根系分泌物介导下植物-土壤-微生物互作关系研究进展与展望[J].植物生态学报,2014,38(3):298-310. |
| WU L K, LIN X M, LIN W X. Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates [J]. Chin. J. Plant Ecol., 2014, 38(3):298-310. |
| [1] | 杨莉, 于俐, 孙卓, 张桐毓, 张阳, 杨利民. 人参根系分泌物中有机酸及皂苷对人参病原菌与生防菌的化感差异研究[J]. 中国农业科技导报, 2022, 24(6): 145-155. |
| [2] | 戴良香, 张冠初, 丁红, 徐扬, 张智猛. 有机肥和钙肥对盐碱土花生根际细菌群落结构的影响[J]. 中国农业科技导报, 2022, 24(5): 189-201. |
| [3] | 王方玲, 张明月, 周亚茹, 管庆林, 李欣燕, 钟秋, 赵铭钦. 干旱胁迫下TS-PAA保水剂对雪茄烟生长发育和光合特性的影响[J]. 中国农业科技导报, 2022, 24(4): 162-172. |
| [4] | 李江艳, 张鲜花, 袁小强. 鸭茅种质资源苗期抗旱指标筛选及抗旱评价[J]. 中国农业科技导报, 2022, 24(3): 84-94. |
| [5] | 张微, 李志新, 赵雪, 张金鹏, 付春江, 刘卫平, 于倩倩. 同时快速检测马铃薯X病毒、Y病毒和S病毒试纸条的研制[J]. 中国农业科技导报, 2022, 24(1): 211-217. |
| [6] | 孙晓春, 黄文静, 李铂. 水杨酸对干旱胁迫下桔梗幼苗生理生化指标及相关基因表达的影响[J]. 中国农业科技导报, 2022, 24(1): 63-70. |
| [7] | 李成晨, 索海翠, 罗焕明, 安康, 刘计涛, 王丽, 单建伟, 杨少海, 李小波. 化肥减施和施肥方式对马铃薯产量和块茎氮素积累的影响[J]. 中国农业科技导报, 2021, 23(9): 173-182. |
| [8] | 于显枫, 张绪成, 缪平贵, 方彦杰, 马一凡, 王红丽, 侯慧芝. 深施肥对立式深旋耕马铃薯水分利用效率及产量的影响[J]. 中国农业科技导报, 2021, 23(7): 182-190. |
| [9] | 刘源, 张秀妍, 徐妙云, 郑红艳, 邹俊杰, 张兰, 王磊. 水稻干旱胁迫的small RNA转录组分析[J]. 中国农业科技导报, 2021, 23(6): 23-32. |
| [10] | 胡杨, 李钢铁, 李星, 贾守义. 干旱胁迫对细穗柽柳幼苗生长和生理生化指标的影响[J]. 中国农业科技导报, 2021, 23(6): 43-50. |
| [11] | 张豪洋,金伊楠,孙燕鑫,李子玮,郭笑恒,许自成*. 植物microRNAs在干旱胁迫响应中的研究进展[J]. 中国农业科技导报, 2021, 23(4): 27-36. |
| [12] | 王得运1,2,刘培培1,陈云婷1,徐月莹1,周丽1,罗光明1*. 干旱胁迫对栀子内源激素含量的影响[J]. 中国农业科技导报, 2021, 23(4): 58-63. |
| [13] | 杨华1,李江2,张维1,周正富1,燕永亮1,郭嘉3,刘相国3,郝东云3,林敏1,柯秀彬1*. 施氏假单胞菌在玉米根际的固氮效率和促生效果研究[J]. 中国农业科技导报, 2021, 23(4): 76-84. |
| [14] | 苏雨萌§,张旭婷§,特日格乐,田敏,尚晓蕊,李国婧,王瑞刚*. 高通量测序鉴定中间锦鸡儿干旱条件下的microRNA[J]. 中国农业科技导报, 2021, 23(3): 51-57. |
| [15] | 张志东1,顾美英1,唐琦勇1,楚敏1,朱静1,孙建1,杨蓉1,徐万里2*. 盐爪爪根际耐盐促生菌的筛选及穴栽验证[J]. 中国农业科技导报, 2021, 23(3): 186-192. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号