




















中国农业科技导报 ›› 2024, Vol. 26 ›› Issue (7): 147-155.DOI: 10.13304/j.nykjdb.2023.0651
• 动植物健康 • 上一篇
谢勇俊( ), 潘小卓, 陈福慧, 尹凯波, 金嘉悦, 王一兵(
), 潘小卓, 陈福慧, 尹凯波, 金嘉悦, 王一兵( )
)
收稿日期:2023-08-30
接受日期:2024-02-06
出版日期:2024-07-15
发布日期:2024-07-12
通讯作者:
王一兵
作者简介:谢勇俊 E-mail:jun146ban@163.com;
基金资助:
Yongjun XIE( ), Xiaozhuo PAN, Fuhui CHEN, Kaibo YIN, Jiayue JIN, Yibing WANG(
), Xiaozhuo PAN, Fuhui CHEN, Kaibo YIN, Jiayue JIN, Yibing WANG( )
)
Received:2023-08-30
Accepted:2024-02-06
Online:2024-07-15
Published:2024-07-12
Contact:
Yibing WANG
摘要:
随着人参种植年限的增加,土壤中自毒物质的积累会导致连作障碍的发生,极大地影响人参种植业的健康发展。生物降解土壤中自毒物质是缓解连作障碍的有效途径。以酚酸类自毒物质为筛选指标,从人参根际土壤中分离、筛选酚酸类自毒物质降解细菌,结合16S rRNA基因测序及生理生化试验对降解菌株进行分类鉴定,采用紫外分光光度法测定其降解能力,并进一步采用单因素试验对其培养条件进行优化,利用降解菌对酚酸胁迫下的人参种子进行生防研究。结果表明,从人参根际土壤中分离出10株自毒物质降解菌,以假单胞菌属(Pseudomonas)为主。初步降解试验显示菌株S1对水杨酸的降解率最高,达65.32%,经鉴定该菌株为伯克霍尔德属(Burkholderia)细菌。单因素试验结果表明,以硝酸钙作为氮源,培养温度30 ℃,500 mg·L-1自毒物质下,菌株S1的降解率达88.58%,较优化前明显提升。生防试验结果表明,菌株S1可缓解水杨酸对人参种子生长的抑制作用,促生效率达12.56%。综上所述,从土壤中分离出可降解水杨酸的伯克霍尔德属菌株S1具有较好的生防效果,对于解决连作障碍问题具有潜在生防应用价值。
中图分类号:
谢勇俊, 潘小卓, 陈福慧, 尹凯波, 金嘉悦, 王一兵. 人参酚酸类自毒物质降解菌的筛选鉴定及生防研究[J]. 中国农业科技导报, 2024, 26(7): 147-155.
Yongjun XIE, Xiaozhuo PAN, Fuhui CHEN, Kaibo YIN, Jiayue JIN, Yibing WANG. Screening, Identification and Biocontrol of Bacteria Degrading Ginseng Phenolic Acid Autotoxic Substances[J]. Journal of Agricultural Science and Technology, 2024, 26(7): 147-155.
培养基 Culture medium | 组分 Component |
|---|---|
筛选培养基 Screening medium | 1 L培养基含(NH2)SO₄ 2 g、CaCl₂ 0.1 g、NaH2PO4 0.5 g、K2HPO4 0.5 g、MgSO4·7H2O 0.2 g、碳源(水杨酸、没食子酸、邻苯二甲酸、对羟基苯甲酸、丁香酸、香草酸、肉桂酸、魏酸)500 mg,1 000 mL蒸馏水,pH 7.2,固体培养基加入20 g琼脂粉 1 L medium contains 2 g (NH2)SO₄, 0.1 g CaCl₂, 0.5 g NaH2PO4, 0.5 g K2HPO4, 0.2 g MgSO4·7H2O, 500 mg carbon source (salicylic acid, gallic acid, phthalic acid, p-hydroxybenzoic acid, butyric acid, vanillic acid, cinnamic acid and ferulic acid), 1 000 mL water,and pH 7.2, the solid medium contains 20 g agar powder |
Hugh-Leifson 培养基 Hugh-Leifson medium | 1 L培养基含葡萄糖10 g,蛋白胨2 g,磷酸氢二钾0.3 g,氯化钠5 g,琼脂3~5 g, 质量浓度为1% 溴麝香草酚蓝3 mL 1 L medium contains 10 g glucose, 2 g peptone, 0.3 g dipotassium hydrogen phosphate, 5 g sodium chloride, 3~5 g agar and 3 mL 1% bromomuscohol blue by mass |
明胶培养基 Gelatin medium | 1 L培养基含蛋白胨5 g,牛肉膏3 g,明胶120 g,pH 6.8~7.0 1 L medium contains 5 g peptone, 3 g beef paste, 120 g gelatin, pH 6.8~7.0 |
硝酸盐培养基 Nitrate medium | 1 L培养基含KNO3(不含NO2-)0.2 g,蛋白胨5 g,pH 7.4 1 L medium contains 0.2 g KNO3 (NO2- free), 5 g peptone, pH 7.4 |
尿素培养基 Urea medium | 1 L培养基含尿素20.0 g,Na2HPO4 9.5 g,酵母浸膏0.1 g,酚红0.01 g,KH2PO4 9.1 g, pH (6.8±0.2) 1 L medium contains 20.0 g urea, 9.5 g Na2HPO4, 0.1 g yeast extract, 0.01 g phenol red, 9.1 g KH2PO4, and pH (6.8±0.2) |
LB培养基 LB medium | 1 L培养基含胰蛋白胨10 g、酵母提取物5 g、氯化钠10 g 1 L medium contains 10 g tryptone, 5 g yeast extract, and 10 g sodium chloride |
表1 试验所需培养基配方
Table 1 Test the required medium formulation
培养基 Culture medium | 组分 Component |
|---|---|
筛选培养基 Screening medium | 1 L培养基含(NH2)SO₄ 2 g、CaCl₂ 0.1 g、NaH2PO4 0.5 g、K2HPO4 0.5 g、MgSO4·7H2O 0.2 g、碳源(水杨酸、没食子酸、邻苯二甲酸、对羟基苯甲酸、丁香酸、香草酸、肉桂酸、魏酸)500 mg,1 000 mL蒸馏水,pH 7.2,固体培养基加入20 g琼脂粉 1 L medium contains 2 g (NH2)SO₄, 0.1 g CaCl₂, 0.5 g NaH2PO4, 0.5 g K2HPO4, 0.2 g MgSO4·7H2O, 500 mg carbon source (salicylic acid, gallic acid, phthalic acid, p-hydroxybenzoic acid, butyric acid, vanillic acid, cinnamic acid and ferulic acid), 1 000 mL water,and pH 7.2, the solid medium contains 20 g agar powder |
Hugh-Leifson 培养基 Hugh-Leifson medium | 1 L培养基含葡萄糖10 g,蛋白胨2 g,磷酸氢二钾0.3 g,氯化钠5 g,琼脂3~5 g, 质量浓度为1% 溴麝香草酚蓝3 mL 1 L medium contains 10 g glucose, 2 g peptone, 0.3 g dipotassium hydrogen phosphate, 5 g sodium chloride, 3~5 g agar and 3 mL 1% bromomuscohol blue by mass |
明胶培养基 Gelatin medium | 1 L培养基含蛋白胨5 g,牛肉膏3 g,明胶120 g,pH 6.8~7.0 1 L medium contains 5 g peptone, 3 g beef paste, 120 g gelatin, pH 6.8~7.0 |
硝酸盐培养基 Nitrate medium | 1 L培养基含KNO3(不含NO2-)0.2 g,蛋白胨5 g,pH 7.4 1 L medium contains 0.2 g KNO3 (NO2- free), 5 g peptone, pH 7.4 |
尿素培养基 Urea medium | 1 L培养基含尿素20.0 g,Na2HPO4 9.5 g,酵母浸膏0.1 g,酚红0.01 g,KH2PO4 9.1 g, pH (6.8±0.2) 1 L medium contains 20.0 g urea, 9.5 g Na2HPO4, 0.1 g yeast extract, 0.01 g phenol red, 9.1 g KH2PO4, and pH (6.8±0.2) |
LB培养基 LB medium | 1 L培养基含胰蛋白胨10 g、酵母提取物5 g、氯化钠10 g 1 L medium contains 10 g tryptone, 5 g yeast extract, and 10 g sodium chloride |
| 编号Number | 比对结果Alignment result | 相似度Similarity/% |
|---|---|---|
| 14XC | 铜绿假单胞菌Pseudomonas aeruginosa | 99.52 |
| 19AW | Pseudomonas furukawaii | 99.67 |
| BH1AW | Pandoraea morbifera | 99.87 |
| BH2XC | 木糖氧化无色杆菌Achromobacter xylosoxidans | 99.55 |
| D2 | Pseudomonas qingdaonensis | 99.86 |
| DXH1 | 德莱无色杆菌Achromobacter deleyi | 99.22 |
| L1 | Methylorubrum populi | 99.85 |
| MSZ | 涅斯特连科氏菌Nesterenkonia | 99.72 |
| S1 | 伯克霍尔德菌Burkholderia FNTGs | 100.00 |
| RG1 | 副伯克霍尔德氏菌Paraburkholderia phytofirmans | 99.86 |
表2 基于16S rRNA的序列比对
Table 2 Sequence alignment based on 16S rRNA gene
| 编号Number | 比对结果Alignment result | 相似度Similarity/% |
|---|---|---|
| 14XC | 铜绿假单胞菌Pseudomonas aeruginosa | 99.52 |
| 19AW | Pseudomonas furukawaii | 99.67 |
| BH1AW | Pandoraea morbifera | 99.87 |
| BH2XC | 木糖氧化无色杆菌Achromobacter xylosoxidans | 99.55 |
| D2 | Pseudomonas qingdaonensis | 99.86 |
| DXH1 | 德莱无色杆菌Achromobacter deleyi | 99.22 |
| L1 | Methylorubrum populi | 99.85 |
| MSZ | 涅斯特连科氏菌Nesterenkonia | 99.72 |
| S1 | 伯克霍尔德菌Burkholderia FNTGs | 100.00 |
| RG1 | 副伯克霍尔德氏菌Paraburkholderia phytofirmans | 99.86 |
| 项目Item | 结果Result | 项目Item | 结果Result |
|---|---|---|---|
| 革兰氏染色Gram stainning method | - | 硝酸盐还原试验Nitratereduction test | - |
| 吲哚试验Indole test | - | 明胶液化试验Gelatinliquefaction test | - |
| 甲基红试验Methylred test | + | 尿素酶试验Urease test | + |
| V⁃P试验 V⁃P test | + | 氧化-发酵试验Oxidation⁃fermentation test | + |
表3 菌株S1的生理生化检测结果
Table 3 Physiological and biochemical test results of strain S1
| 项目Item | 结果Result | 项目Item | 结果Result |
|---|---|---|---|
| 革兰氏染色Gram stainning method | - | 硝酸盐还原试验Nitratereduction test | - |
| 吲哚试验Indole test | - | 明胶液化试验Gelatinliquefaction test | - |
| 甲基红试验Methylred test | + | 尿素酶试验Urease test | + |
| V⁃P试验 V⁃P test | + | 氧化-发酵试验Oxidation⁃fermentation test | + |
酚酸种类 Type of phenolic acid | 标准曲线 Standard curve | 检测波长 Wave length/nm | 菌株 Strain | 降解率 Degradation rate/% |
|---|---|---|---|---|
| 水杨酸Salicylic acid | y=0.005 74x-0.011 91,R2=0.995 0 | 300 | S1 | 65.32 |
| 香草酸Vanillic acid | y=0.006 57x+0.041 87,R2=0.996 2 | 260 | 14XC | 55.00 |
| 丁香酸Syringic acid | y=0.004 47x+0.035,R2=0.990 8 | 280 | DXH1 | 48.00 |
对羟基苯甲酸 Para-hydroxybenzoic acid | y=0.107 90x-0.019 97,R2=0.999 7 | 225 | D2 | 43.00 |
| 邻苯二甲酸Phthalic acid | y=0.004 19x+0.001 86,R2=0.998 9 | 216 | L1 | 41.37 |
| 肉桂酸Cinnamic acid | y=0.016 66x-0.039 72,R2=0.990 8 | 278 | RG1 | 34.00 |
表4 6株人参自毒物质降解菌的降解效果
Table 4 Degradation effects of six strains of ginseng autotoxic substances degrading bacteria
酚酸种类 Type of phenolic acid | 标准曲线 Standard curve | 检测波长 Wave length/nm | 菌株 Strain | 降解率 Degradation rate/% |
|---|---|---|---|---|
| 水杨酸Salicylic acid | y=0.005 74x-0.011 91,R2=0.995 0 | 300 | S1 | 65.32 |
| 香草酸Vanillic acid | y=0.006 57x+0.041 87,R2=0.996 2 | 260 | 14XC | 55.00 |
| 丁香酸Syringic acid | y=0.004 47x+0.035,R2=0.990 8 | 280 | DXH1 | 48.00 |
对羟基苯甲酸 Para-hydroxybenzoic acid | y=0.107 90x-0.019 97,R2=0.999 7 | 225 | D2 | 43.00 |
| 邻苯二甲酸Phthalic acid | y=0.004 19x+0.001 86,R2=0.998 9 | 216 | L1 | 41.37 |
| 肉桂酸Cinnamic acid | y=0.016 66x-0.039 72,R2=0.990 8 | 278 | RG1 | 34.00 |
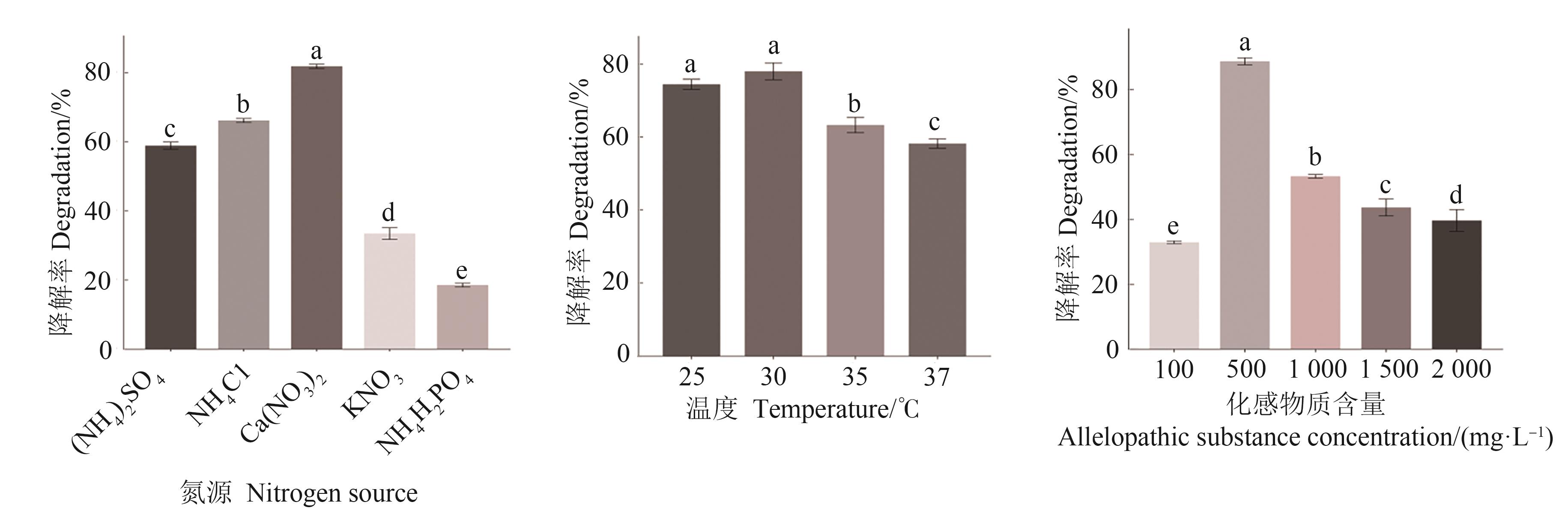
图3 不同培养条件下菌株S1对水杨酸的降解率注:不同小写字母表示在P<0.05水平显著差异。
Fig. 3 Degradation rate of strain S1on salicylic acid under different cultured conditionsNote:Different lowercase letter indicate significant differences at P<0.05 level.
处理 Treatment | 胚根长 Radicle length /mm | 胚轴长/mm Hypocotyl length /mm | RI胚根长 RI of radicle length | RI胚轴长 RI of hypocotyl length |
|---|---|---|---|---|
| CK | 53.42±5.70 a | 27.95±8.76 a | — | — |
| SA0.025 | 46.87±12.04 ab | 13.57±4.16 bc | -0.128±0.18 b | -0.514±0.03 b |
| SA2.5 | 46.61±3.58 ab | 12.30±1.01 bc | -0.124±0.06 b | -0.539±0.10 b |
| SA250 | 35.77±4.55 b | 7.10±1.60 c | -0.330±0.05 b | -0.731±0.10 c |
| SA250+S1 | 60.13±8.71 a | 18.31±2.89 b | 0.154±0.13 a | -0.145±0.02 a |
表5 不同处理下人参种子的胚根和胚轴长
Table 5 Radicle and hypocotyl lengths of ginseng seed under different treatments
处理 Treatment | 胚根长 Radicle length /mm | 胚轴长/mm Hypocotyl length /mm | RI胚根长 RI of radicle length | RI胚轴长 RI of hypocotyl length |
|---|---|---|---|---|
| CK | 53.42±5.70 a | 27.95±8.76 a | — | — |
| SA0.025 | 46.87±12.04 ab | 13.57±4.16 bc | -0.128±0.18 b | -0.514±0.03 b |
| SA2.5 | 46.61±3.58 ab | 12.30±1.01 bc | -0.124±0.06 b | -0.539±0.10 b |
| SA250 | 35.77±4.55 b | 7.10±1.60 c | -0.330±0.05 b | -0.731±0.10 c |
| SA250+S1 | 60.13±8.71 a | 18.31±2.89 b | 0.154±0.13 a | -0.145±0.02 a |
| 1 | 张益恺,初赛君,董亚南,等.简述长白山人参在大健康产业中的应用[J].人参研究,2023,35(1):34-37. |
| 2 | 孙仁爽,赵敏婧,隋艳艳,等.人参药材抗氧化活性的研究[J].人参研究,2023,35(3):28-31. |
| 3 | BLOK W J, BOLLEN G J. The role of autotoxins from root residues of the previous crop in the replant disease of Asparagus [J]. Netherlands J. Plant Pathol., 1993, 99(3):29-40. |
| 4 | BENNETT J A, KLIRONOMOS J. Mechanisms of plant-soil feedback: interactions among biotic and abiotic drivers [J]. New Phytol., 2018, 222(1):91-96. |
| 5 | 周亭亭,战宇,李琼,等.人参属药用植物化感物质种类及其作用机制研究进展[J/OL].特产研究,2023:1-6 [2023-07-20].. |
| ZHOU T T, ZHAN Y, LI Q, et al.. Research progress on the types and mechanism of allelochemicals in ginseng medicinal plant [J/OL]. Spec. Res., 2023: 1-6 [2023-07-20]. . | |
| 6 | 李自博.人参根系自毒物质在连作障碍中的化感作用及其缓解途径研究[D].沈阳:沈阳农业大学,2018. |
| LI Z B. Allelopathy of autotoxic compounds and mitigation method for ginseng continuous cropping obstacle [D]. Shenyang: Shenyang Agricultural University, 2018. | |
| 7 | HE C N, GAO W W, YANG J X, et al.. Identification of autotoxic compounds from fibrous roots of Panax quinquefolium L. [J]. Plant Soil, 2009, 318(1/2):63-72. |
| 8 | 陈福慧,谢勇俊,贾清文,等.林下连作种植参根际土壤可培养微生物区系及细菌群落对酚酸类化感物质的响应[J].广西科学,2023,30(3):468-477. |
| CHEN F H, XIE Y J, JIA Q W, et al.. Response of culturable microflora and bacterial community to phenolic allelochemica in rhizosphere soil of continuous cropping Panax ginseng C. A. Meyer under forest [J]. Guangxi Sci., 2023, 30(3):468-477. | |
| 9 | 刘莹,孙文松,李玲,等.人参连作障碍及防治措施研究进展[J].园艺与种苗,2020,40(7):26-29. |
| LIU Y, SUN W S, LI L, et al.. Research progress on consecutive monoculture problems and control measures of Panax ginseng [J]. Hortic. Seed, 2020, 40(7):26-29. | |
| 10 | 向维,韦小兰,曹科鑫,等.三七皂苷类自毒物质降解细菌分离及其降解特性[J].广西植物,2023,43(7):1173-1181. |
| XIANG W, WEI X L, CAO K X, et al.. Isolation and characterization of autotoxic saponins-degrading bacterial strains from Panax notoginseng [J]. Guihaia, 2023, 43(7):1173-1181. | |
| 11 | 王罗涛,杨冬英,邓琳梅,等.三七根际土壤中皂苷类自毒物质降解拮抗细菌的分离筛选[J].南方农业学报,2020,51(2):305-312. |
| WANG L T, YANG D Y, DENG L M, et al.. Isolation and screening of antagonistic autotoxin-degrading bacteria in Panax notoginseng (Burk.) F. H. Chen rhizosphere soil [J]. J. South Agric., 2020, 51(2):305-312. | |
| 12 | 赵亚慧,华雪洁,杜海岩,等.花生化感物质降解菌和抗连作拮抗菌复合菌剂应用效果的研究[J].土壤通报,2016,47(3):599-604. |
| ZHAO Y H, HUA X J, DU H Y, et al.. Research on the application effect of compound bacterium agent for allelochemicals degradation bacteria and antagonistic bacteria resistance to continuous cropping [J]. Chin. J. Soil Sci., 2016, 47(3):599-604. | |
| 13 | 张娜.微生物菌剂对设施番茄连作障碍的防治效果[J].蔬菜,2022(11):29-31. |
| ZHANG N. Effects of microbial inoculants on control of continuous cropping obstacles of facilities tomato [J]. Vegetables, 2022(11):29-31. | |
| 14 | 毛宁,薛泉宏,唐明,等.放线菌对对羟基苯甲酸的降解作用及草莓生长的影响[J].中国农业科技导报,2010,12(5):103-108. |
| MAO N, XUE Q H, TANG M, et al.. Degradation of para-hydroxybenzoic acid by actinom yces and its effects on strawberry growth [J]. J. Agric. Sci. Technol., 2010, 12(5):103-108. | |
| 15 | 肖蓉,邓舒,赵菁,等.自毒物质对羟基苯甲酸降解细菌ZH2的分离与应用[J].农学学报,2021,11(7):84-91. |
| XIAO R, DENG S, ZHAO J, et al.. An autotoxicity p-hydroxybenzoic acid-degrading strain ZH2: isolation and application [J]. J. Agron., 2021, 11(7):84-91. | |
| 16 | 东秀珠,蔡妙英.常见细菌系统鉴定手册[M].北京:科学出版社,2001:66-127. |
| 17 | GIBBONS N E, BUCHANAN R E. 伯杰细菌鉴定手册[M].北京:科学出版社,1984:446-450. |
| 18 | BRUCE-WILLIAMSON G, RICHARDSON D R. Bioassays for allelopathy: measuring treatment responses with independent controls [J]. J. Chem. Ecol., 1988, 14 (1):181-187. |
| 19 | 崔丙健,崔二苹,刘春成,等.土壤改良剂对再生水滴灌根际土壤菌群多样性及病原菌和抗生素抗性基因丰度的影响[J].环境科学,2022,43(10):4765-4778. |
| CUI B J, CUI E P, LIU C C, et al.. Effects of soil amendments on the bacterial diversity and abundances of pathogens and antibiotic resistance genes in rhizosphere soil under drip irrigation with reclaimed water [J]. Environ. Sci., 2022, 43(10):4765-4778. | |
| 20 | WANG X Q, CHEN D X, WANG J, et al.. Cloning and analysis of genes controlling antibacterial activities of Burkholderia pyrrocinia strain Lyc2 [J]. Curr. Microbiol., 2019, 76:1003-1009. |
| 21 | 许玉. Tsukamurella tyrosinosolvens P9、Burkholderia pyrrocinia P10与花生互作机制的比较研究[D].贵阳:贵州大学,2022. |
| XU Y. Comparative study on the interaction mechanism between Tsukamurella tyrosinosolvens P9, Burkholderia pyrrocinia P10 and peanut [D]. Guiyang: Guizhou University, 2022. | |
| 22 | HAN L, ZHANG H, XU Y, et al.. Biological characteristics and salt-tolerant plant growth-promoting effects of an ACC deaminase-producing Burkholderia pyrrocinia strain isolated from the tea rhizosphere [J]. Arch. Microbiol., 2021, 203:2279-2290. |
| 23 | 王鹏飞.丹参自毒物质的鉴定及其在腐解液和根际土中的含量分析[D].泰安:山东农业大学,2021. |
| WANG P F. Identification of autotoxic substances from Salvia miltiorrhiza and analysis of their contents in decomposed solution and rhizosphere soil [D]. Tai’an: Shandong Agricultural University, 2021. | |
| 24 | 张博洋,陈彦宏,栗锦鹏,等.自毒物质降解菌缓解药用植物连作障碍的作用及机制研究进展[J].中国野生植物资源,2023,42(11):7-14. |
| ZHANG B Y, CHEN Y H, LI J P, et al.. Research progress on the role and mechanisms of autotoxicity-degrading bacteria in alleviating continuous cropping obstacles of medicinal plants [J]. Chin. Wild Plant Res., 2023, 42(11):7-14. | |
| 25 | 李敏,张丽叶,张艳江,等.酚酸类自毒物质微生物降解转化研究进展[J].生态毒理学报,2019,14(3):72-78. |
| LI M, ZHANG L Y, ZHANG Y J, et al.. Review on the microbial biodegradation and metabolism of autotoxic phenolic acids [J]. Asian J. Ecotoxicol., 2019, 14(3):72-78. | |
| 26 | PEETERS C, ZLOSNIK J E A, SPILKER T, et al.. Burkholderia pseudomultivorans sp. nov., a novel Burkholderia cepacia complex species from human respiratory samples and the rhizosphere [J]. Syst. Appl. Microbiol., 2013, 36(7):483-489. |
| 27 | WILHELM R C, DERITO C M, SHAPLEIGH J P, et al.. Phenolic acid-degrading Paraburkholderia prime decomposition in forest soil [J/OL]. Cold Spring Harbor Lab., 2020, 9: 317347 [2023-07-20]. . |
| [1] | 周喜新, 袁世林, 杨柳, 夏滔, 张毅, 范伟. 连作烟草根系分泌物鉴定及潜在化感物质的筛选研究[J]. 中国农业科技导报, 2024, 26(7): 136-146. |
| [2] | 施琳波, 彭晴, 徐小轻, 张宇微, 杨硕, 田丹丹, 何孟欣, 石波, 乔宇. 共培养干酪乳杆菌NA-2和鼠李糖乳杆菌LGG对抑菌活性的影响[J]. 中国农业科技导报, 2024, 26(6): 63-71. |
| [3] | 张桐毓, 勾颖, 李琪, 杨莉. 人参锈腐病对人参品质和土壤相关因子的影响研究[J]. 中国农业科技导报, 2024, 26(3): 124-133. |
| [4] | 王艳成, 张纪月, 冯帅奇, 梁雪, 张振, 董微巍, 姬文秀. 外源促生菌联合有机肥对干旱胁迫下参地土壤性状及人参抗逆性影响[J]. 中国农业科技导报, 2023, 25(8): 196-202. |
| [5] | 李小玲, 周武先, 蒋小刚, 李大荣, 黄大野, 张美德. 微生物菌肥对川党参连作障碍及紫纹羽病的防控效果[J]. 中国农业科技导报, 2023, 25(3): 119-131. |
| [6] | 胡茜, 王艺凝, 申鹏飞, 李雅倩, 杨阳, 王艳成, 姬文秀, 董微巍. 洋虫内产β-葡萄糖苷酶内生菌发酵液转化人参皂苷产物分析及其抗肿瘤活性研究[J]. 中国农业科技导报, 2023, 25(2): 119-127. |
| [7] | 闫宁, 战宇, 苗馨月, 王二刚, 陈长宝, 李琼. 强还原土壤灭菌处理对人参连作土壤细菌群落结构及土壤酶活的影响[J]. 中国农业科技导报, 2022, 24(6): 133-144. |
| [8] | 杨莉, 于俐, 孙卓, 张桐毓, 张阳, 杨利民. 人参根系分泌物中有机酸及皂苷对人参病原菌与生防菌的化感差异研究[J]. 中国农业科技导报, 2022, 24(6): 145-155. |
| [9] | 陈福慧, 申乃坤, 姜明国, 王一兵. 作物重茬连作障碍中自毒物质的研究进展[J]. 中国农业科技导报, 2022, 24(10): 125-132. |
| [10] | 李舒欣, 张浩, 郑厚胜, 郑培和, 逄世峰, 许世泉. 转录组分析二马牙和长脖类型林下参表型差异[J]. 中国农业科技导报, 2021, 23(9): 56-68. |
| [11] | 李浩成1,2,左应梅2,杨绍兵2,杨天梅2,李纪潮2,杨维泽2,张金渝2*. 三七根系分泌物在连作障碍中的生态效应及缓解方法[J]. 中国农业科技导报, 2020, 22(8): 159-167. |
| [12] | 符可芯1,2,杨叶2*,曾耿狄1. 热带土壤中解淀粉芽孢杆菌HNU1的鉴定及发酵条件优化[J]. 中国农业科技导报, 2020, 22(6): 49-59. |
| [13] | 李庆凯1,2,3,刘苹2,3*,赵海军3,宋效宗2,林海涛2,沈玉文2,李林1,万书波1,3*. 玉米根系分泌物对连作花生土壤酚酸类物质化感作用的影响[J]. 中国农业科技导报, 2020, 22(3): 119-130. |
| [14] | 李海涛1,黄曦漫1,李玉1,刘广娜1,奚广生2*. 人参单体皂苷对桃蚜取食、解毒酶及乙酰胆碱酯酶活性的影响[J]. 中国农业科技导报, 2019, 21(11): 103-110. |
| [15] | 蔡小雨1,闫培生1*,高秀君1,陈琪琪1,郭长禄1,刘润东2,梁浩2,张明臣2. 人参皂苷生物转化的研究进展[J]. 中国农业科技导报, 2018, 20(4): 52-60. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号