




















Journal of Agricultural Science and Technology ›› 2023, Vol. 25 ›› Issue (5): 66-76.DOI: 10.13304/j.nykjdb.2021.0657
• BIOTECHNOLOGY & LIFE SCIENCE • Previous Articles Next Articles
Xiaoting WANG( ), Pengpeng ZHANG(
), Pengpeng ZHANG( )
)
Online:2023-05-20
Published:2023-07-13
Contact:
Pengpeng ZHANG
About author:WANG XiaotingE-mail: xiaotingwang2021@163.com
Supported by:通讯作者:
张芃芃
CLC Number:
Xiaoting WANG, Pengpeng ZHANG. Phylogenic and Functional Profile of Ser/Thr Kinases in Synechocystis sp. PCC 6803[J]. Journal of Agricultural Science and Technology, 2023, 25(5): 66-76.
王小婷, 张芃芃. 集胞藻6803中丝氨酸/苏氨酸激酶系统发育和功能概述[J]. 中国农业科技导报, 2023, 25(5): 66-76.
| Gene product | Gene ID | Category |
|---|---|---|
| SpkA | sll 1574~1575 | PKN2 subfamily |
| SpkB | slr 1697 | PKN2 subfamily |
| SpkC | slr 0599 | PKN2 subfamily |
| SpkD | sll 0776 | PKN2 subfamily |
| SpkE | slr 1443 | PKN2 subfamily |
| SpkF | slr 1225 | PKN2 subfamily |
| SpkG | slr 0152 | PKN2 subfamily |
| SpkH | sll 0005 | ABC1 subfamily |
| SpkI | sll 1770 | ABC1 subfamily |
| SpkJ | slr 0889 | ABC1 subfamily |
| SpkK | slr 1919 | ABC1 subfamily |
| SpkL | sll 0095 | ABC1 subfamily |
Table 1 12 Ser/Thr kinases in Synechocystis sp. PCC 6803
| Gene product | Gene ID | Category |
|---|---|---|
| SpkA | sll 1574~1575 | PKN2 subfamily |
| SpkB | slr 1697 | PKN2 subfamily |
| SpkC | slr 0599 | PKN2 subfamily |
| SpkD | sll 0776 | PKN2 subfamily |
| SpkE | slr 1443 | PKN2 subfamily |
| SpkF | slr 1225 | PKN2 subfamily |
| SpkG | slr 0152 | PKN2 subfamily |
| SpkH | sll 0005 | ABC1 subfamily |
| SpkI | sll 1770 | ABC1 subfamily |
| SpkJ | slr 0889 | ABC1 subfamily |
| SpkK | slr 1919 | ABC1 subfamily |
| SpkL | sll 0095 | ABC1 subfamily |
| Gene | Function description |
|---|---|
| spkA | Involved in cell motility[ |
| spkB | Participate in the oxidative stress response[ |
| spkC | Participate in the regulation of nitrogen metabolism[ |
| spkD | Involved in adjusting the pool of TCA cycle metabolites[ |
| spkE | Respond to cold stress[ |
| spkF | Involved in the phosphorylation of the GroES chaperone protein[ |
| spkG | Involved in high-salt acclimation[ |
| spkH | Involved in hyperosmotic stress response[ |
| spkK | Involved in the phosphorylation of the GroES chaperone protein[ |
Table 2 Partly published function descriptions among 12 Ser/Thr kinases in Synechocystis sp. PCC 6803
| Gene | Function description |
|---|---|
| spkA | Involved in cell motility[ |
| spkB | Participate in the oxidative stress response[ |
| spkC | Participate in the regulation of nitrogen metabolism[ |
| spkD | Involved in adjusting the pool of TCA cycle metabolites[ |
| spkE | Respond to cold stress[ |
| spkF | Involved in the phosphorylation of the GroES chaperone protein[ |
| spkG | Involved in high-salt acclimation[ |
| spkH | Involved in hyperosmotic stress response[ |
| spkK | Involved in the phosphorylation of the GroES chaperone protein[ |
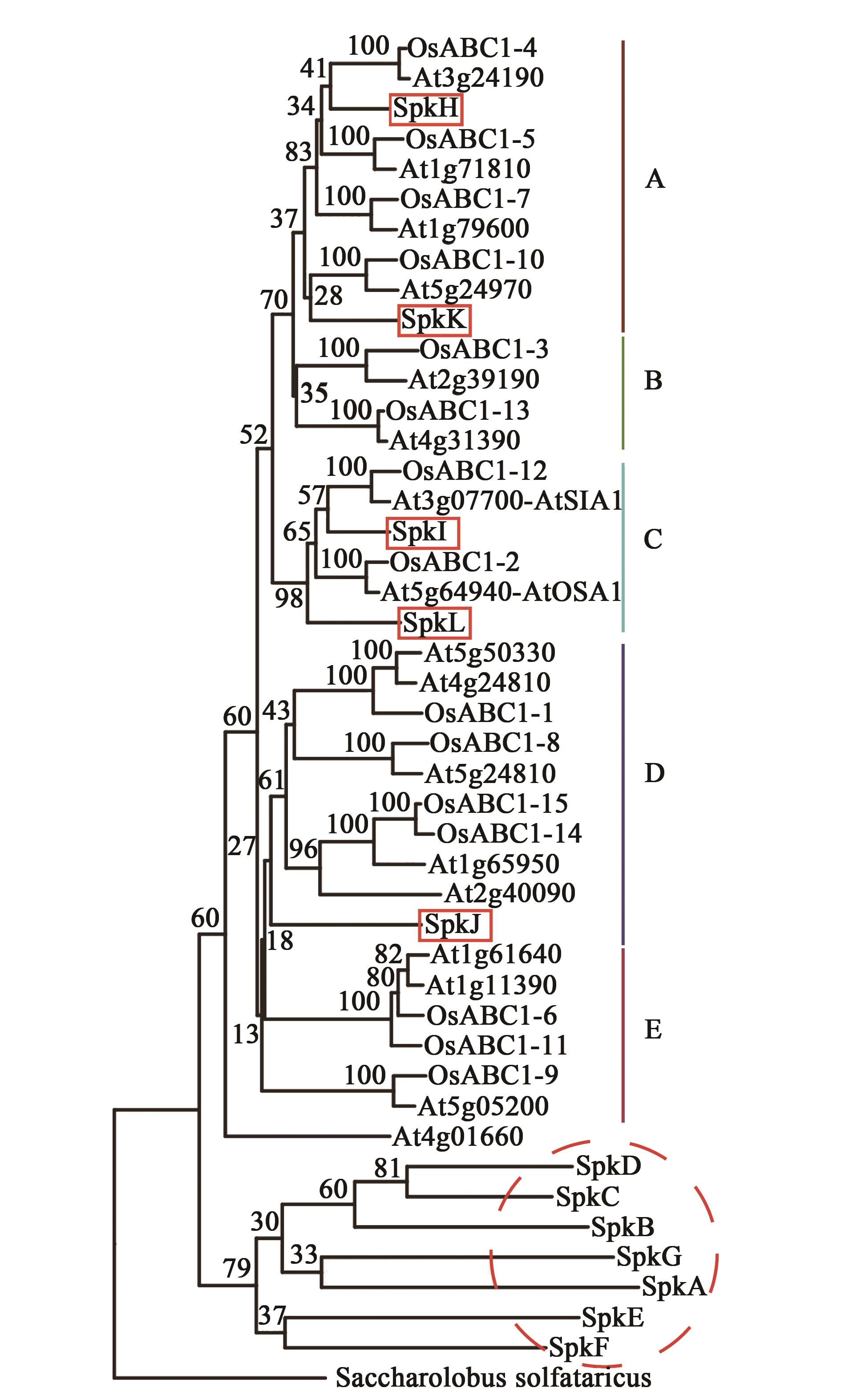
Fig. 1 Phylogenetic analysis of Ser/Thr kinases in Synechocystis sp. PCC 6803, rice and ArabidopsisNote:12 Ser/Thr kinases in Synechocystis sp. PCC 6803 used for construction of phylogenetic tree are listed in Table 1, amino acid sequences are derived from KEGG GENES database (https://www.genome.jp/kegg/genes.html); 15 ABC1Ks in rice are from RiceData; 17 ABC1Ks in Arabidopsis are derived from the Arabidopsis information resource(https://arabidopsis.org/). The rooted tree is generated using MEGA6 program by neighbor-joining method. The scale bar indicates 0.2 amino acid substitutions per synonymous site.

Fig. 2 Motif predictions of 12 Ser/Thr kinases in Synechocystis sp. PCC 6803Note:Amino acid sequences are retrieved from KEGG GENES database (https://www.genome.jp/kegg/genes.html); motif prediction is conducted on MEME website (https://meme-suite.org/).
| Gene product | Rice | Arabidopsis | ||||
|---|---|---|---|---|---|---|
| Homolog | Location | Predicted function | Homolog | Location | Predicted function | |
| SpkH | OsABC1-4 | Chloroplast | Cold stress[ | At3g24190 | Chloroplast | Protein phosphorylation |
| SpkK | OsABC1-10 | Mitochondria | Salt stress[ | At5g24970 | Mitochondria | Protein phosphorylation |
| SpkI | OsABC1-12 | Chloroplast | Cold and salt stress; biosynthesis of isoprenoids[ | At3g07700 (AtSIA1; ABC1K7) | Chloroplast | Salt stress[ |
| SpkL | OsABC1-2 | Chloroplast | Salt, H2O2 and darkness; energy metabolism[ | At5g64940 (AtOSA1; ABC1K8) | Chloroplast | Cadmium and oxidative stresses; isoprenyl lipid synthesis; distribution of iron within chloroplast[ |
| SpkJ | OsABC1-14, -15 | Vacuole | H2O2, cold, drought, and darkness[ | At1g65950 (ABC1K14); At2g40090 (ABC1K15) | Extracellular region | Lipid homeostasis; protein phosphorylation |
Table 3 Homology of 5 Ser/Thr kinases (SpkH-SpkL)in rice and Arabidopsis, respectively
| Gene product | Rice | Arabidopsis | ||||
|---|---|---|---|---|---|---|
| Homolog | Location | Predicted function | Homolog | Location | Predicted function | |
| SpkH | OsABC1-4 | Chloroplast | Cold stress[ | At3g24190 | Chloroplast | Protein phosphorylation |
| SpkK | OsABC1-10 | Mitochondria | Salt stress[ | At5g24970 | Mitochondria | Protein phosphorylation |
| SpkI | OsABC1-12 | Chloroplast | Cold and salt stress; biosynthesis of isoprenoids[ | At3g07700 (AtSIA1; ABC1K7) | Chloroplast | Salt stress[ |
| SpkL | OsABC1-2 | Chloroplast | Salt, H2O2 and darkness; energy metabolism[ | At5g64940 (AtOSA1; ABC1K8) | Chloroplast | Cadmium and oxidative stresses; isoprenyl lipid synthesis; distribution of iron within chloroplast[ |
| SpkJ | OsABC1-14, -15 | Vacuole | H2O2, cold, drought, and darkness[ | At1g65950 (ABC1K14); At2g40090 (ABC1K15) | Extracellular region | Lipid homeostasis; protein phosphorylation |
| ID | Gene product | Peptide length | Number of predicted TM helix | Start and end position |
|---|---|---|---|---|
| slr0599 | SpkC | 535 | 1 | 344~366 |
| sll0776 | SpkD | 505 | 1 | 326~348 |
| slr1225 | SpkF | 495 | 3 | 372~394, 399~416, 463~485 |
| sll1770 | SpkI | 585 | 2 | 530~549, 553~575 |
| sll0095 | SpkL | 567 | 3 | 27~46, 503~525, 530~552 |
Table 4 Transmembrane helix prediction of Ser/Thr kinases in Synechocystis sp. PCC 6803
| ID | Gene product | Peptide length | Number of predicted TM helix | Start and end position |
|---|---|---|---|---|
| slr0599 | SpkC | 535 | 1 | 344~366 |
| sll0776 | SpkD | 505 | 1 | 326~348 |
| slr1225 | SpkF | 495 | 3 | 372~394, 399~416, 463~485 |
| sll1770 | SpkI | 585 | 2 | 530~549, 553~575 |
| sll0095 | SpkL | 567 | 3 | 27~46, 503~525, 530~552 |
| Gene product | Cellular compartment | Intensity (A. U.) | ||||
|---|---|---|---|---|---|---|
| OM | PM1 | PM2 | TM | SOL | ||
| SpkB | 3.5 | 0.3 | 0.5 | 0.1 | / | 1×107 |
| SpkC | 0.9 | 3 | 4.3 | 0.3 | / | 1×107 |
| SpkE | 0.7 | 0.3 | 1.9 | / | / | 1×106 |
| SpkF | 0.2 | 0.6 | 0.9 | 0.2 | / | 1×107 |
| SpkH | 0.1 | 1.3 | 0.4 | 2.1 | / | 1×107 |
| SpkI | / | 3.7 | 1.9 | 1.4 | / | 1×107 |
| SpkJ | 3.3 | 3.3 | 1.4 | / | / | 1×105 |
| SpkK | / | 1.9 | 0.2 | 2.5 | 0.3 | 1×107 |
| SpkL | / | 2.8 | 1.9 | 1.1 | 0.2 | 1×106 |
Table 5 Subcellular localization prediction of Ser/Thr kinases in Synechocystis sp. PCC 6803
| Gene product | Cellular compartment | Intensity (A. U.) | ||||
|---|---|---|---|---|---|---|
| OM | PM1 | PM2 | TM | SOL | ||
| SpkB | 3.5 | 0.3 | 0.5 | 0.1 | / | 1×107 |
| SpkC | 0.9 | 3 | 4.3 | 0.3 | / | 1×107 |
| SpkE | 0.7 | 0.3 | 1.9 | / | / | 1×106 |
| SpkF | 0.2 | 0.6 | 0.9 | 0.2 | / | 1×107 |
| SpkH | 0.1 | 1.3 | 0.4 | 2.1 | / | 1×107 |
| SpkI | / | 3.7 | 1.9 | 1.4 | / | 1×107 |
| SpkJ | 3.3 | 3.3 | 1.4 | / | / | 1×105 |
| SpkK | / | 1.9 | 0.2 | 2.5 | 0.3 | 1×107 |
| SpkL | / | 2.8 | 1.9 | 1.1 | 0.2 | 1×106 |
| 1 | DUTTA R, QIN L, INOUYE M. Histidine kinases: diversity of domain organization [J]. Mol. Microbiol., 1999, 34(4): 633-640. |
| 2 | ALEX L A, SIMON M I. Protein kinase and signal transduction in prokaryotes and eukaryotes [J]. Trends Genet., 1994, 10(4): 133-138. |
| 3 | STOCK A M, ROBINSON V L, GOUDREAU P N. Two-component signal transduction [J]. Annu. Rev. Biochem., 2000, 69:183-215. |
| 4 | KHORCHID A, IKURA M. Bacterial histidine kinase as signal sensor and transducer [J]. Int. J. Biochem. Cell Biol., 2006, 38(3): 307-312. |
| 5 | HANKS S K, HUNTER T. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification1 [J]. FASEB J., 1995, 9(8): 576-596. |
| 6 | HUNTER T. Signaling—2000 and beyond [J]. Cell, 2000, 100(1): 113-127. |
| 7 | MU OZ-DORADO J, INOUYE S, INOUYE M. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium [J]. Cell, 1991, 67(5): 995-1006. |
| 8 | ZHANG C C. Bacterial signaling involving eukaryotic-type protein kinases [J]. Mol. Microbiol., 1996, 20(1): 9-15. |
| 9 | KENNELLY P J, POTTS M. Fancy meeting you here! a fresh look at “prokaryotic” protein phosphorylation [J]. J. Bacteriol., 1996, 178(16): 4759-4764. |
| 10 | RIPPKA R. Isolation and purification of cyanobacteria [J]. Methods Enzymol., 1988, 167: 3-27. |
| 11 | MANN N H. Protein phosphorylation in cyanobacteria [J]. Microbiology, 1995, 140 (Pt 12): 3207-3215. |
| 12 | ZHANG X W, ZHAO F Q, GUAN X Y, et al.. Genome-wide survey of putative serine/threonine protein kinases in cyanobacteria [J/OL]. BMC Genomics, 2007, 8(1): 395 [2021-07-09]. . |
| 13 | YANG M K, QIAO Z X, ZHANG W Y, et al. . Global phosphoproteomic analysis reveals diverse functions of serine/threonine/tyrosine phosphorylation in the model cyanobacterium Synechococcus sp strain PCC 7002 [J]. J. Proteome Res., 2013, 12(4): 1909-1923. |
| 14 | CHEN Z, ZHAN J, CHEN Y, et al.. Effects of phosphorylation of beta subunits of phycocyanin on state transition in the model cyanobacterium Synechocystis sp PCC 6803 [J]. Plant Cell Physiol., 2015, 56(10): 1997-2013. |
| 15 | SPÄT P, MAČEK B, FORCHHAMMER K. Phosphoproteome of the cyanobacterium Synechocystis sp. PCC 6803 and its dynamics during nitrogen starvation [J/OL]. Front. Microbiol., 2015, 6: 248 [2021-07-09]. . |
| 16 | ANGELERI M, MUTH-PAWLAK D, ARO E M, et al.. Study of O-phosphorylation sites in proteins involved in photosynthesis-related processes in Synechocystis sp. strain PCC 6803: application of the SRM approach [J]. J. Proteome Res., 2016,15(12): 4638-4652. |
| 17 | ZHANG C C, JANG J C, SAKR S, et al.. Protein phosphorylation on Ser, Thr and Tyr residues in cyanobacteria [J]. J. Mol. Microbiol. Biotechnol., 2006, 9(3-4): 154-166. |
| 18 | KANEKO T, SATO S, KOTANI H, et al.. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. Ⅱ. Sequence determination of the entire genome and assignment of potential protein-coding regions [J]. DNA Res., 1996, 3(3): 109-136. |
| 19 | WILLIAMS J G K. Construction of specific mutations in PSII photosynthetic reaction center by genetic engineering [J]. Methods Enzymol., 1988,167:766-778. |
| 20 | XU W, WANG Y C. Sequences, domain architectures, and biological functions of the sserine/threonine and histidine kinases in Synechocystis sp. PCC 6803 [J]. Appl. Biochem. Biotechnol., 2019, 188: 1022-1065. |
| 21 | HAN G N, ZHANG C C. On the origin of Ser/Thr kinases in a prokaryote [J]. FEMS Microbiol. Lett., 2001, 200(1): 79-84. |
| 22 | ZORINA A A. Eukaryotic protein kinase in cyanobacteria [J]. Russ. J. Plant Physiol., 2013, 60(5): 589-596. |
| 23 | LEONARD C J, ARAVIND L, KOONIN E V. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily [J]. Genome Res., 1998, 8(10): 1038-1047. |
| 24 | LUNDQUIST P K, POLIAKOV A, GIACOMELLI L, et al.. Loss of plastoglobule kinases ABC1K1 and ABC1K3 causes conditional degreening, modified prenyl-lipids, and recruitment of the jasmonic acid pathway [J]. Plant Cell, 2013, 25(5): 1818-1839. |
| 25 | MANARA A, DALCORSO G, FURINI A. The role of the atypical kinases ABC1K7 and ABC1K8 in abscisic acid responses [J/OL]. Front. Plant Sci., 2016, 7: 366 [2021-07-09]. . |
| 26 | HANKS S K, QUINN A M, HUNTER T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains [J]. Science, 1988, 241(4861): 42-52. |
| 27 | ZHANG C C. A gene encoding a protein related to eukaryotic protein kinases from the filamentous heterocystous cyanobacterium Anabaena PCC 7 120 [J]. Proc. Natl. Acad. Sci. USA, 1994, 90(24): 11840-11844. |
| 28 | MUELLER P R, COLEMAN T R, KUMAGAI A, et al.. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15 [J]. Science, 1995, 270(5233): 86-90. |
| 29 | ZHANG C C, GONZALEZ L, PHALIP V. Survey analysis and genetic organization of genes encoding eukaryotic-like signaling proteins on a cyanobacterial genome [J]. Nucleic Acids Res., 1998, 26(16): 3619-3625. |
| 30 | KAMEI A, YOSHIHARA S, YUASA T, et al.. Biochemical and functional characterization of a eukaryotic-type protein kinase, SpkB, in the cyanobacterium Synechocystis sp. PCC 6803 [J]. Curr. Microbiol., 2003, 46(4): 296-301. |
| 31 | KAMEI A, YUASA T, ORIKAWA K, et al.. A eukaryotic-type protein kinase, SpkA, is required for normal motility of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 [J]. J. Bacteriol., 2001, 183(5): 1505-1510. |
| 32 | PANICHKIN V B, ARAKAWA-KOBAYASHI S, KANASEKI T, et al.. Serine/threonine protein kinase SpkA in Synechocystis sp. strain PCC 6803 is a regulator of expression of three putative pilA operons, formation of thick pili, and cell motility [J]. J. Bacteriol., 2006, 188(21): 7696-7699. |
| 33 | ZORINA A A, STEPANCHENKO N, NOVIKOVA G V, et al.. Eukaryotic-like Ser/Thr protein kinases SpkC/F/K are involved in phosphorylation of GroES in the cyanobacterium Synechocystis [J]. DNA Res., 2011, 18(3): 137-151. |
| 34 | MATA-CABANA A, GARCÍA-DOMÍNGUEZ M, FLORENCIO F J, et al.. Thiol-based redox modulation of a cyanobacterial eukaryotic-type serine/threonine kinase required for oxidative stress tolerance [J]. Antioxid. Redox Signal., 2012, 17(4): 521-533. |
| 35 | SINETOVA M A, LOS D A. New insights in cyanobacterial cold stress responses: genes, sensors, and molecular triggers [J]. Biochim. Biophys. Acta Gen. Subj., 2016, 1860: 2391-2403. |
| 36 | LAURENT S, JANG J, JANICKI A, et al. . Inactivation of spkD, encoding a Ser/Thr kinase, affects the pool of the TCA cycle metabolites in Synechocystis sp. strain PCC 6803 [J]. Microbiology, 2008, 154(7): 2161-2167. |
| 37 | WANG H L, POSTIER B L, BURNAP R L. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator [J]. J. Biol. Chem., 2004, 279(7): 5739-5751. |
| 38 | ZORINA A A, BEDBENOV V S, NOVIKOVA G V, et al. . Involvement of serine/threonine protein kinases in cold stress response in the cyanobacterium Synechocystis sp. PCC 6803: functional characterization of a protein kinase SpkE [J]. Mol. Biol. (Mosk), 2014, 48(3): 452-462. |
| 39 | GALKIN A N, MIKHEEVA L E, SHESTAKOV S V. The insertional inactivation of genes encoding eukaryotic-type serine/threonine protein kinases in the cyanobacterium Synechocystis sp. PCC 6803 [J]. Microbiology, 2003, 72(1): 52-57. |
| 40 | LIANG C W, ZHANG X W, CHI X Y, et al. . Serine/threonine protein kinase SpkG is a candidate for high salt resistance in the unicellular cyanobacterium Synechocystis sp. PCC 6803 [J/OL]. PLoS ONE, 2011, 6(5): e18718 [2021-07-09]. . |
| 41 | ANGELERI M, ZORINA A, ARO E, et al.. Interplay of SpkG kinase and the Slr0151 protein in the phosphorylation of ferredoxin 5 in Synechocystis sp. strain PCC 6803 [J]. FEBS Lett., 2018, 592(3): 411-421. |
| 42 | PAITHOONRANGSARID K, SHOUMSKAYA M A, KANESAKI Y, et al. . Five histidine kinases perceive osmotic stress and regulate distinct sets of genes in Synechocystis [J]. J. Biol. Chem., 2004, 279(51): 53078-53086. |
| 43 | CALZADILLA P I, ZHAN J, SÉTIF P, et al.. The cytochrome b6f complex is not involved in cyanobacterial state transitions [J]. Plant Cell, 2019, 31(4): 911-931. |
| 44 | XU W, WANG Y C. Post-translational modifications of serine/threonine and histidine kinases and their roles in signal transductions in Synechocystis Sp. PCC 6803 [J]. Appl. Biochem. Biotechnol., 2021, 193(3): 687-716. |
| 45 | KAMEI A, YUASA T, GENG X, et al. . Biochemical examination of the potential eukaryotic-type protein kinase genes in the complete genome of the unicellular cyanobacterium Synechocystis sp. PCC 6803 [J]. DNA Res., 2002, 9(3): 71-78. |
| 46 | WANG L, SUN Y P, CHEN W L, et al.. Genomic analysis of protein kinases, protein phosphatases and two-component regulatory systems of the cyanobacterium Anabaena sp. strain PCC 7120 [J]. FEMS Microbiol. Lett., 2002, 217(2): 155-165. |
| 47 | GALKIN A N, MIKHEEVA L E, SHESTAKOV S V. Insertional inactivation of genes encoding eukaryotic type serine/threonine protein kinases in cyanobacterium Synechocystis sp. PCC 6803 [J]. Mikrobiologiia, 2003, 72(1): 64-69. |
| 48 | GAO Q S, ZHANG D, XU L, et al. . Systematic identification of rice ABC1 gene family and its response to abiotic stress [J]. Rice Sci., 2011, 18(3): 167-177. |
| 49 | GAO Q S, ZANG H, GAO Y, et al. . Comprehensive molecular evolution and gene expression analyses of the ABC1 atypical kinase family in rice and Arabidopsis [J]. J. Plant Biochem. Biotechnol., 2015, 24(2): 210-217. |
| 50 | YANG S, ZHANG Q, LI T, et al. . AtSIA1, an ABC1-like kinase, regulates salt response in Arabidopsis [J]. Biologia, 2012, 67(6): 1107-1111. |
| 51 | JASINSKI M, SUDRE D, SCHANSKER G, et al.. AtOSA1, a member of the Abc1-like family, as a new factor in cadmium and oxidative stress response [J]. Plant Physiol., 2008, 147(2): 719-731. |
| 52 | YANG S G, ZENG X Q, LI Tet al.. AtACDO1, an ABC1-like kinase gene, is involved in chlorophyll degradation and the response to photooxidative stress in Arabidopsis [J]. J. Exp. Bot., 2012, 63(10): 3959-3973. |
| 53 | MARTINIS J, GLAUSER G, VALIMAREANU S, et al.. ABC1K1/PGR6 kinase: a regulatory link between photosynthetic activity and chloroplast metabolism [J]. Plant J., 2014, 77(2): 269-283. |
| 54 | MARTINIS J, GLAUSER G, VALIMAREANU S, et al.. A chloroplast ABC1-like kinase regulates vitamin E metabolism in Arabidopsis [J]. Plant Physiol., 2013, 162(2): 652-662. |
| 55 | LUNDQUIST P K, DAVIS J I, VAN WIJK K J. ABC1K atypical kinases in plants: filling the organellar kinase void [J]. Trends Plant Sci., 2012, 17(9): 546-555. |
| [1] | Lu MENG, Jingwen FAN, Xinyu SAI, Lusheng ZENG, Xiangyun SONG, Dejie CUI. Effects of Lime on Soil Improvement and Plant Growth in Apple Orchards [J]. Journal of Agricultural Science and Technology, 2023, 25(4): 197-204. |
| [2] | Ye ZHANG, Hao ZHANG, Pengpeng ZHANG. Interplay of Cyclic Electron Transport and Mehler-like Reaction in Synechocystis Under Different Light Regimes [J]. Journal of Agricultural Science and Technology, 2023, 25(3): 78-95. |
| [3] | Ailin DAI, Yonggang ZHANG, Qiang AI, Xichao TENG, Limin YANG. Effects of Different Light Qualities on Growth, Physiological Characteristics and Flavonoids Accumulation of Epimedium koreanum [J]. Journal of Agricultural Science and Technology, 2022, 24(4): 85-92. |
| [4] | MA Xingdong1, GUO Yehong1*, DU Tao2, LI Meiying3, XU Yingjie1, CHEN Xiaowei1, YAN Zongbang1, YANG Shaojie1, FENG Jinglu1. Effects of Nitrogen Application on Diurnal Variation of Photosynthesis and Yield of Two Lycium Species [J]. Journal of Agricultural Science and Technology, 2021, 23(4): 173-182. |
| [5] | HAO Zhenggang, ZHAO Huijun, WEI Yuqing*, ZENG Zhouqi, WANG Zhiheng. Physiological and Biochemical Responses of Sweet Sorghum to Cadmium Stress and Its Cadmium Accumulation [J]. Journal of Agricultural Science and Technology, 2021, 23(1): 30-42. |
| [6] | YIN Yonggang, YUAN Junwei, LIU Changjiang, HAN Bin, LI Minmin, SUN Yan, JIA Nan, GUO Zijuan, ZHAO Shengjian*. Effects of NaCl Stress on Leaf Photosynthesis and Chlorophyll Fluorescence Parameters of Vitis sp. Rootstocks [J]. Journal of Agricultural Science and Technology, 2020, 22(8): 49-55. |
| [7] | LIU Shidou1, ZHU Xinping1,2*, ZHAO Yi1, WANG Boyan1, HAN Yaoguang1, YANG Beibei1, LU Zhi1, JIA Hongtao1,2. Cotton Stalk Biochar Retarding Stress of Cadmium on Growth of Rice [J]. Journal of Agricultural Science and Technology, 2020, 22(4): 139-146. |
| [8] | LI Meng, CHEN Dong, LI Xiuni, LI Linlin, WANG Ronghao, SHI Xiangdong*. Influenes of Exogenous Melatonin on Antioxidant and Photosynthetic Characteristics of Tobacco Seedlings Under Salt Stress [J]. Journal of Agricultural Science and Technology, 2019, 21(2): 141-147. |
| [9] | WANG Jian, SHI Jing. Prediction Model of SVR Photosynthetic Rate Based on Chemotaxis-Improved Particle Swarm Optimization [J]. Journal of Agricultural Science and Technology, 2018, 20(12): 74-82. |
| [10] | SHEN Ya-jun1, CUI Xue-an2, ZHANG Zhi-guo2, WU Jin-xia2*. Gene Mapping of a Rolled Leaf Mutant rlm1 in Rice [J]. Journal of Agricultural Science and Technology, 2016, 18(2): 25-30. |
| [11] | SONG Yun1, LI Lin-xuan2, ZHUO Feng-ping2, ZHANG Xue-yan1, REN Mao-zhi2, LI Fu-gu. Progress on Jasmonic Acid Signaling in Plant Stress Resistant [J]. , 2015, 17(2): 17-24. |
| [12] | LI Yuan-yuan1,2, CAO Qing-he1,2*. Mechanism of Brassinosteroid Involved in Regulating Plant Development, Stress Resistance and its Application in Breeding [J]. , 2015, 17(2): 25-32. |
| [13] | DUAN Long\|fei1, SHANG Ai\|qin1*, YANG Min\|sheng2, WANG Jin\|mao2, ZUO Li\|hui2. Studies on Diurnal Variations of Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters of Different Clones of Ulmus pumila cv. ‘Zhonghua jinye’ [J]. , 2014, 16(6): 21-27. |
| [14] | FENG Lei, ZHANG Hai-wen*, HUANG Rong-feng. Research Progress on LRR Receptorlike Protein Kinase in Plant [J]. , 2012, 14(6): 43-48. |
| [15] | HAO Yan-hua, ZHANG Wei, CHEN Ming. Research Progress on Two Component System of Bacteria [J]. , 2012, 14(2): 67-72. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号