




















中国农业科技导报 ›› 2025, Vol. 27 ›› Issue (5): 133-145.DOI: 10.13304/j.nykjdb.2023.0152
史硕1( ), 冯宇1(
), 冯宇1( ), 李亮1(
), 李亮1( ), 孟瑞1, 章延泽1, 杨秀荣2
), 孟瑞1, 章延泽1, 杨秀荣2
收稿日期:2023-03-06
接受日期:2023-05-05
出版日期:2025-05-15
发布日期:2025-05-20
通讯作者:
李亮
作者简介:史硕 E-mail:SS17320080170@163.com基金资助:
Shuo SHI1( ), Yu FENG1(
), Yu FENG1( ), Liang LI1(
), Liang LI1( ), Rui MENG1, Yanze ZHANG1, Xiurong YANG2
), Rui MENG1, Yanze ZHANG1, Xiurong YANG2
Received:2023-03-06
Accepted:2023-05-05
Online:2025-05-15
Published:2025-05-20
Contact:
Liang LI
摘要:
小麦纹枯病是一种世界性频发土传病害,对小麦稳产高产危害严重。为了明确内生真菌印度梨形孢(Piriformospora indica)对小麦抗纹枯病的诱导作用,突破传统依赖于化学药剂的防治模式,利用生物方法提高小麦对纹枯病的抗性,采用印度梨形孢孢子液浸泡小麦种子,然后用禾谷丝核菌(Rhizoctonia cerealis)侵染小麦植株,并对其相关生理生化指标和转录组数据进行分析。结果表明,小麦预先定殖印度梨形孢能增加细胞内抗氧化酶活性,缓解由于病菌引起的细胞内相对水含量降低,使因病菌侵染而破环的的膜稳定性上升39.6%,并能提高32.3%的叶绿素含量,增强小麦光合作用。转录组分析结果显示,印度梨形孢的定殖能改变病原菌引起的转录组变化并诱导相关抗性基因表达,通过影响激素代谢途径,提高植物生物量,利用多种路径和手段综合提高小麦对禾谷丝核菌的抗性。基于转录组数据筛选出了TraesCS1A02G372300、TraesCS1B02G393100等关键基因,为深入理解植物与微生物互作、加速相关抗病增产育种工作等提供理论基础与试验依据。
中图分类号:
史硕, 冯宇, 李亮, 孟瑞, 章延泽, 杨秀荣. 印度梨形孢介导小麦抗纹枯病的转录组分析及关键基因筛选[J]. 中国农业科技导报, 2025, 27(5): 133-145.
Shuo SHI, Yu FENG, Liang LI, Rui MENG, Yanze ZHANG, Xiurong YANG. Transcriptome Analysis of Resistance to Sharp Eyespot of Wheat Mediated by Piriformospora indica and Key Genes Screening[J]. Journal of Agricultural Science and Technology, 2025, 27(5): 133-145.
处理 Treatment | 重复 Repeat | 总干净片段 Total clean read/106 | 对比到基因组的总比对率 Total mapping genome ratio/% | 对比到基因组的唯一比对率 Uniquely mapping genome ratio/% |
|---|---|---|---|---|
| Mock | 3 | 88.57 | 75.83 | 19.69 |
| Piri | 3 | 88.94 | 75.07 | 20.03 |
| Rh | 3 | 88.31 | 78.25 | 18.85 |
| Piri+ Rh | 3 | 88.64 | 77.87 | 19.93 |
表1 不同样品的处理
Table 1 Respective treatments for different sample
处理 Treatment | 重复 Repeat | 总干净片段 Total clean read/106 | 对比到基因组的总比对率 Total mapping genome ratio/% | 对比到基因组的唯一比对率 Uniquely mapping genome ratio/% |
|---|---|---|---|---|
| Mock | 3 | 88.57 | 75.83 | 19.69 |
| Piri | 3 | 88.94 | 75.07 | 20.03 |
| Rh | 3 | 88.31 | 78.25 | 18.85 |
| Piri+ Rh | 3 | 88.64 | 77.87 | 19.93 |
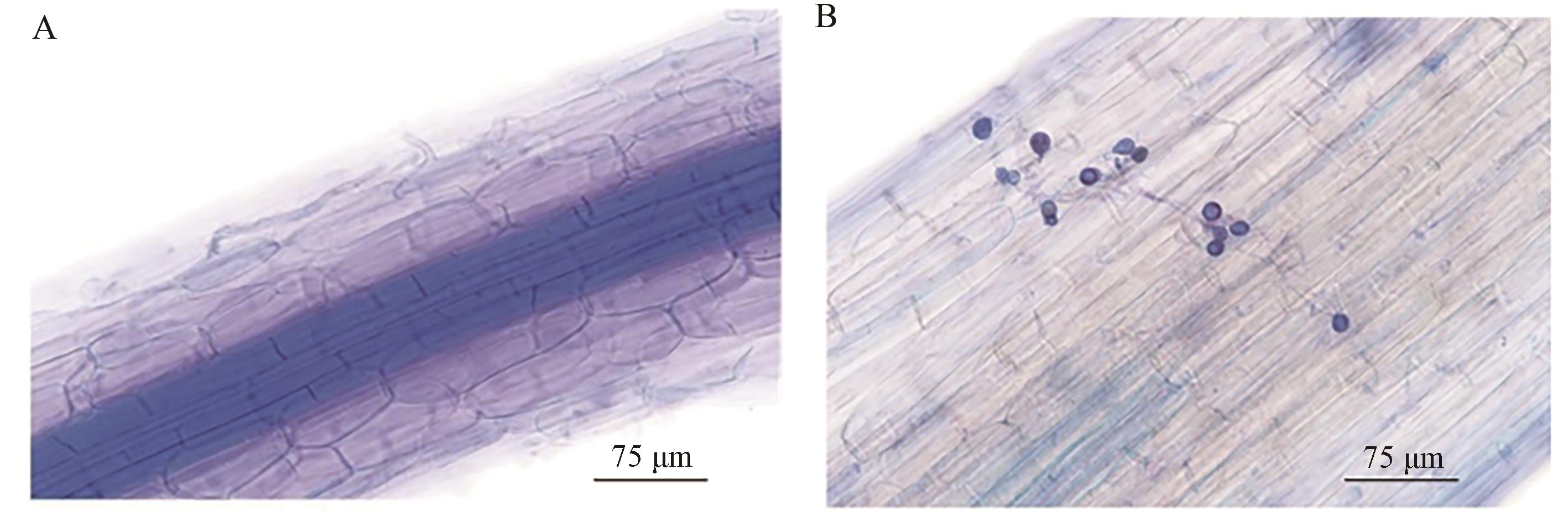
图1 印度梨形孢在小麦根部的定殖A: 吐温-20溶液浸泡组(对照组); B: 孢子液浸泡组(处理组)
Fig. 1 Identification of P. indica colonization in wheat rootA: Control group soaking in Tween-20 solution; B: Treatment group soaking in P. indica
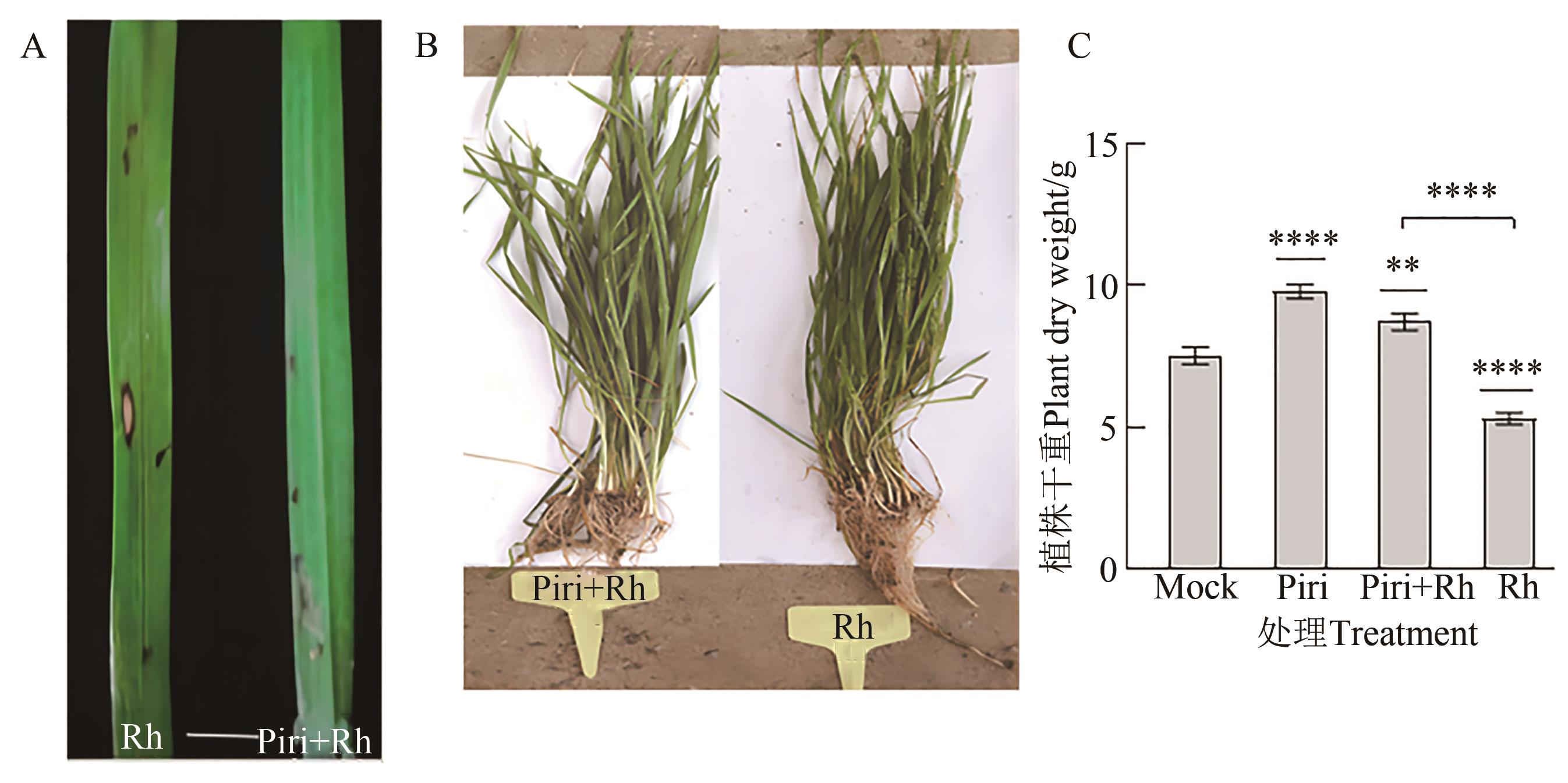
图2 印度梨形孢定殖后的植株及其生物量A: 侵染14 d后Rh和Piri+Rh组的小麦叶片; B: 持续观察60 d后,Rh和Piri+Rh组的植株; C: 各处理组100棵植株的干重。柱形上方星号代表各处理组与对照组之间的差异水平,柱间星号代表不同处理组间的差异水平;**和****分别代表在P<0.01和P<0.000 1水平差异显著
Fig. 2 Plant and its biomass after colonization of P. indicaA: Wheat leaves of Rh and Piri+Rh groups after 14 d of infection; B: Wheat plants of Rh and Piri+Rh groups after 60 d of continuous observation; C: Dry weight of 100 plants among treatment groups. The asterisks above the columns represent the level of difference between each treatment group and the control group, and the asterisks between the columns represent the level of difference between different treatment groups; ** and **** represent significance differences at P<0.01 and P<0.000 1 levels, respectively
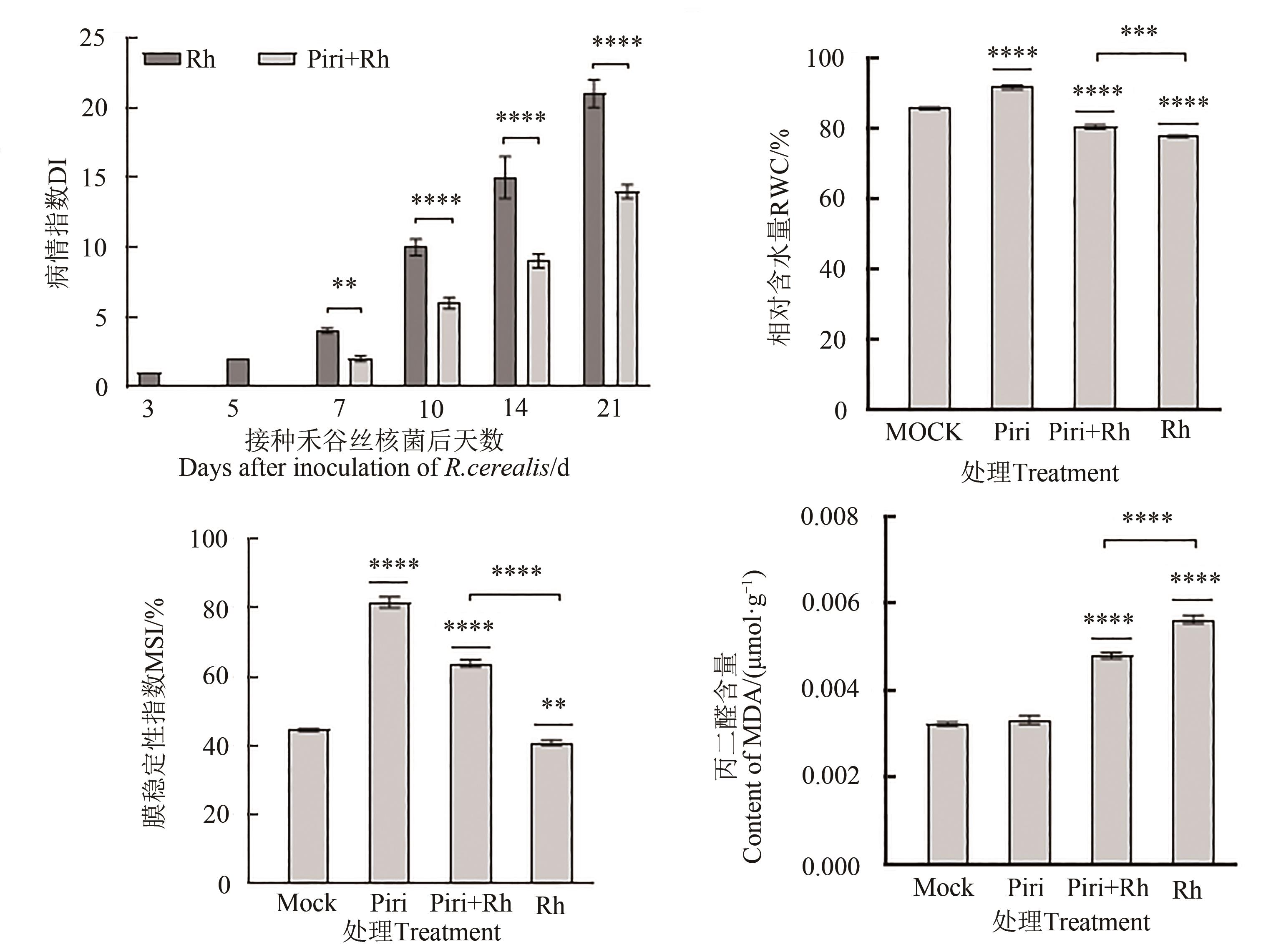
图3 印度梨形孢定殖后小麦的病情指数、叶片相对含水量、膜稳定性指数及丙二醛含量注:柱形上方星号代表各处理组与对照组之间的差异水平,柱间星号代表不同处理组间的差异水平;**、***和****分别代表在P<0.01、P<0.001和 P<0.000 1水平差异显著。
Fig. 3 Disease index,leaf relative water content,membrane stability index and MDA content of wheat after colonization of P. indicaNote: The asterisks above the columns represent the level of difference between each treatment group and the control group, and the asterisks between the columns represent the level of difference between different treatment groups; ** ,*** and **** represents significance differences at P<0.01, P<0.001 and P<0.000 1 levels, respectively.
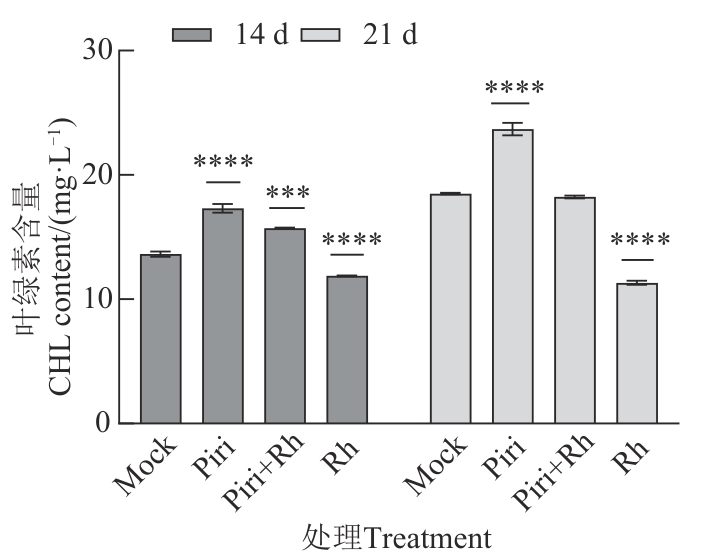
图5 接种禾谷丝核菌14和21 d后叶片中的叶绿素含量注:***和****分别表示与对照间在P<0.001和 P<0.000 1水平差异显著。
Fig. 5 Chlorophyll content of leaves after inoculation of R.cerealis for 14 and 21 dNote:*** and **** represents significance differences between control group at P<0.001 and P<0.000 1 levels,respectively.

图6 不同处理组中差异基因韦恩图以及火山图A: 差异基因数量的韦恩图; B: Rh 和Mock组差异基因的火山图; C: Piri 和Mock组差异基因的火山图; D: Piri+Rh 和Mock组差异基因的火山图
Fig. 6 Venn Diagram and Volcano Plot of different expressed genes among different treatment groupsA: Venn Diagram of the number of different expressed genes; B: Volcano Plot of different expressed genes in Rh and Mock group; C: Volcano Plot of different expressed genes in Piri and Mock group; D: Volcano Plot of different expressed genes in Piri+Rh and Mock group

图7 不同处理组信号通路的富集气泡图A: Rh 和 Mock组中差异基因在KEGG中富集的通路;B: Piri + Rh 和 Mock组中差异基因在KEGG中富集的通路
Fig. 7 Enrichment Bubble Plot of signal pathway in different treatment groupsA: DEGs enriched in KEEG pathway in Rh and Mock groups; B: DEGs enriched in KEEG pathway in Piri + Rh and Mock groups

图8 不同处理组GO富集分析柱状图A: Rh 和 Mock组中差异基因在GO中的富集情况;B: Piri + Rh 和 Mock组中差异基因在GO中的富集情况
Fig. 8 Histogram of GO enrichment analysis for different treatment groupsA: DEGs enriched in GO pathway in Rh and Mock groups; B: DEGs enriched in GO pathway in Piri + Rh and Mock groups
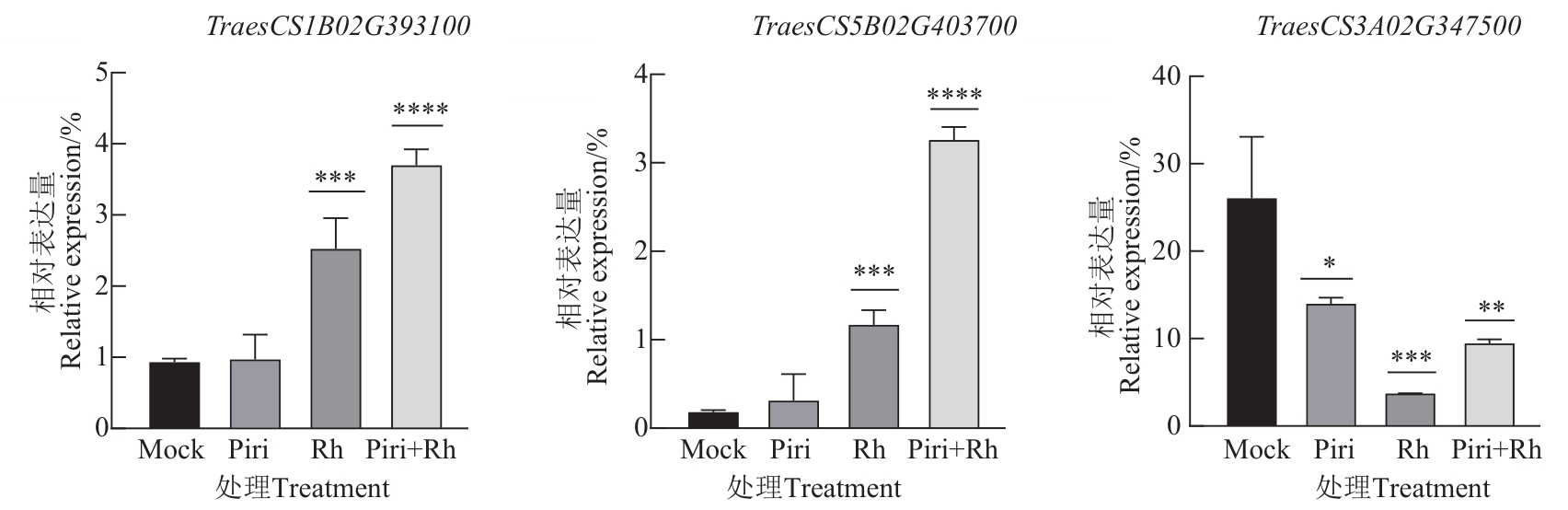
图9 部分关键基因的相对表达量注:*、**、***和****分别表示与对照组在P<0.05、 P<0.01、 P<0.001和P<0.000 1水平差异显著。
Fig. 9 Relative expression of certain key genes by qPCRNote: * , ** ,***and****indicate significance differences between control group at P<0.05, P<0.01, P<0.001 and P<0.000 1 levels,respectively.
| 1 | 孔令让. 另辟蹊径破解小麦条锈病的基因密码[J]. 植物学报, 2022, 57(4): 405-408. |
| KONG L R. Breaking the gene code conferring broad-spectrum resistance to rust fungi [J]. Chin. Bull. Bot., 2022, 57(4): 405-408 | |
| 2 | CHEN L, ZHANG Z Y, LIANG H X, et al.. Overexpression of TiERF1 enhances resistance to sharp eyespot in transgenic wheat [J]. J. Exp. Bot., 2008, 59(15): 4195-4204. |
| 3 | 徐英. 河南小麦根茎部病害种类及防控措施[J]. 农业工程技术, 2018(4): 28-31. |
| 4 | REN X X, CHEN C, YE Z H, et al.. Development and application of seed coating agent for the control of major soil-borne diseases infecting wheat [J/OL]. Agronomy, 2019, 9(8): 413 [2023-02-06]. . |
| 5 | 曹巧,高振贤,单子龙,等. 河北省小麦主要气传病害发生现状及防控对策[J]. 中国农学通报, 2020, 36(6): 100-105. |
| CAO Q, GAO Z X, SHAN Z L, et al.. Status and control countermeasures of major wheat aero-borne diseases in Hebei [J]. Chin. Agric. Sci. Bull., 2020, 36(6): 100-105. | |
| 6 | 卢德鹏,吴远征,扈进冬,等. 木霉菌水分散粒剂防治小麦纹枯病的田间药效研究[J]. 山东科学, 2018, 31(2): 32-35. |
| LU D P, WU Y Z, HU J D, et al.. Field experiment for control of wheat Rhizoctonia cerealis by Trichoderma water-dispersible granules [J]. Shandong Sci., 2018, 31(2): 32-35. | |
| 7 | 张敬敬,汪敏,赵港伊,等. 枯草芽胞杆菌 Z-14 菌株芽孢制剂和酷拉斯复配对小麦纹枯病的防治[J]. 农业生物技术学报, 2023, 31(1): 146-155. |
| ZHANG J J, WANG M, ZHAO G Y, et al.. Control of wheat sharp eyespot by combination of Bacillus subtilis Z-14 spore preparation and kuras [J]. J. Agric. Biotechnol., 2023, 31(1): 146-155. | |
| 8 | ZHU X L, RONG W, WANG K, et al.. Overexpression of TaSTT3b-2B improves resistance to sharp eyespot and increases grain weight in wheat [J]. Plant Biotechnol. J., 2022, 20: 777-739. |
| 9 | LYU G G, ZHANG Y X, MA L, et al.. A cell wall invertase modulates resistance to fusarium crown rot and sharp eyespot in common wheat [J]. J. Integr. Plant Biol., 2023, 65(7):1814-1825. |
| 10 | QI H J, ZHU X L, SHEN W B, et al.. A novel wall-associated kinase TaWAK-5D600 positively participates in defense against sharp eyespot and fusarium crown rot in wheat [J/OL]. Int. J. Mol. Sci., 2023, 24(5): 5060 [2023-02-06]. . |
| 11 | RODRIGUEZ R J, WHITE JR J F, ARNOLD A E, et al.. Fungal endophytes: diversity and functional roles [J]. New Phytol., 2009, 182: 314-330. |
| 12 | BACKMAN P A, SIKORA R A. Endophytes: an emerging tool for biological control [J]. Biol. Control, 2008, 46(1): 1-3. |
| 13 | GILL S S, GILL R, TRIVEDI D K, et al.. Piriformospora indica: potential and significance in plant stress tolerance [J/OL]. Front. Microbiol., 2016, 7: 332 [2023-02-06]. . |
| 14 | 许凤来,朱志炎,何勇,等. 印度梨形孢对铁皮石斛种子萌发和原球茎生长的影响[J].热带亚热带植物学报, 2021, 29 (1): 59-66. |
| XU F L, ZHU Z Y, HE Y, et al.. Effects of Piriformospora indica on seed germination and protocorm growth of Dendrobium officinale [J]. J. Trop. Subtrop. Bot., 2021, 29 (1): 59-66. | |
| 15 | OPITZ M, DANESHKHAH R, LORENZ C, et al.. Serendipita indica changes host sugar and defense status in Arabidopsis thaliana: cooperation or exploitation [J/OL]. Planta, 2021, 253: 74 [2023-02-06]. . |
| 16 | KUNDU A, MISHRA S, KUNDU P, et al.. Piriformospora indica recruits host-derived putrescine for growth promotion in plants [J]. Plant Physiol., 2022, 188(4): 2289-2307. |
| 17 | LI D, BODJRENOU D M, ZHANG S, et al.. The endophytic fungus Piriformospora indica reprograms banana to cold resistance [J/OL]. Int. J. Mol. Sci., 2021, 22(9): 4973 [2023-02-06]. . |
| 18 | GHORBANI A, TAFTEH M, ROUDBARI N, et al.. Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots [J/OL]. Ecotoxicol. Environ. Saf., 2021, 209: 111793 [2023-02-06]. . |
| 19 | 惠非琼,刘剑,高其康,等. 印度梨形孢对烟草抗旱性的影响[J]. 烟草科技, 2017, 50 (12): 1-7. |
| HUI F Q, LIU J, GAO Q K, et al.. Effects of Piriformospora indica on drought resistance of Nicotiana tabacum [J]. Tob. Sci. Technol., 2017, 50 (12): 1-7. | |
| 20 | HARRACH B D, BALTRUSCHAT H, BARNA B, et al.. The mutualistic fungus Piriformospora indica protects barley roots from a loss of antioxidant capacity caused by the necrotrophic pathogen Fusarium culmorum [J]. Mol. Plant Microbe Interact., 2013, 26(5): 599-605. |
| 21 | BAGHAIE A H, AGHILI F. Contribution of Piriformospora indica on improving the nutritional quality of greenhouse tomato and its resistance against cu toxicity after humic acid addition to soil [J]. Environ. Sci. Pollut. Res., 2021, 28(45): 64572-64585. |
| 22 | ROYLAWAR P, KHANDAGALE K, RANDIVE P, et al.. Piriformospora indica primes onion response against stemphylium leaf blight disease [J/OL]. Pathogens, 2021, 10(9): 1085 [2023-02-06]. . |
| 23 | YAN C, MUHAMMAD RIZWAN H, LIANG D, et al.. The effect of the root-colonizing Piriformospora indica on passion fruit (Passiflora edulis) development: initial defense shifts to fitness benefits and higher fruit quality [J/OL]. Food Chem., 2021, 359: 129671 [2023-02-06]. . |
| 24 | STEIN E, MOLITOR A, KOGEL K H, et al.. Systemic resistance in Arabidopsis conferred by the Mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1 [J]. Plant Cell Physiol., 2008, 49: 1747-1751. |
| 25 | TRZEWIK A, MACIOROWSKI R, KLOCKE E, et al.. The influence of Piriformospora indica on the resistance of two rhododendron cultivars to Phytophthora cinnamomi and P.plurivora [J]. Biol. Control, 2020, 140: 104-121. |
| 26 | FAKHRO A, ANDRADE LINARES D R, VON BARGEN S, et al.. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens [J]. Mycorrhiza, 2010, 20(3): 191-200. |
| 27 | QI H, ZHU X, GUO F, et al.. The wall-associated receptor-like kinase TaWAK7D is required for defense responses to Rhizoctonia cerealis in wheat [J/OL]. Int. J. Mol. Sci., 2021, 22(11): 5629 [2023-02-06]. . |
| 28 | ZHU X L, YANG K, WEI X N, et al.. The wheat AGC kinase TaAGC1 is a positive contributor to host resistance to the necrotrophic pathogen Rhizoctonia cerealis [J]. J. Exp. Bot., 2015, 66(21): 6591-603. |
| 29 | WANG X. Principles and Techniques of Plant Physiological Biochemical Experiment [M]. 2nd edition. Beijing: Higher Education Press, 2006:105-109. |
| 30 | 史树德, 孙亚卿, 魏磊. 植物生理学实验指导[M]. 北京: 中国林业出版社, 2011:1-163. |
| 31 | 王妍, 胡胜, 付文成, 等. 一种快速测定可溶性糖的新方法: TBA法[J]. 井冈山大学学报(自然科学版), 2013, 34(3): 37-40. |
| WANG Y, HU S, FU W C, et al.. A new method for fast determination of total soluble sugar content in plant tissue: TBA-method [J]. J. Jinggangshan Univ. (Nat. Sci.), 2013, 34(3): 37-40. | |
| 32 | 杨兰芳, 庞静, 彭小兰, 等. 紫外分光光度法测定殖物过氧化氢酶活性[J]. 现代农业科技, 2009(20): 364-366. |
| YANH L F, PANG J, PENG X L, et al.. Measurement of catalase activity in plants by ultraviolet spectrophotometry [J]. Mod. Agric. Sci. Technol., 2009(20): 364-366. | |
| 33 | 高俊凤. 植物生理学实验指导[M]. 北京: 高等教育出版社, 2006: 214-216. |
| 34 | CHANCE B, MAEHLY A C. Assay of catalases and peroxidases [J]. Meth. Enzymol., 1955, 2: 764-775. |
| 35 | 李志丹, 韩瑞宏, 廖桂兰, 等. 植物叶片中叶绿素提取方法的比较研究[J]. 广东第二师范学院学报, 2011, 31(3): 80-83. |
| LI Z D, HAN R H, LIAO G L, et al.. A comparative study on the different extraction techniques about the chlorophyll concentration of plant leaf [J]. J. Guangdong Univ. Edu., 2011, 31(3): 80-83. | |
| 36 | ROLLI E, MARASCO R, VIGANI G, et al.. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait: root bacteria protect plants from drought [J]. Environ. Microbiol., 2015, 17(2): 316-331. |
| 37 | AN C, MA S J, LIU C, et al.. Burkholderia ambifaria XN08: a plant growth-promoting endophytic bacterium with biocontrol potential against sharp eyespot in wheat [J/OL]. Front. Microbiol., 2022, 13: 906724 [2023-02-06]. . |
| 38 | SHERAMETI I, SHAHOLLARI B, VENUS Y, et al.. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme Glucan-water Dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters [J]. J. Biol. Chem., 2005, 280(28): 26241-26247. |
| 39 | SCHÄFER P, PFIFFI S, VOLL L M, et al.. Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica [J]. Plant J., 2009, 59(3):461-474. |
| 40 | KHATABI B, MOLITOR A, LINDERMAYR C, et al.. Ethylene supports colonization of plant roots by the mutualistic fungus Piriformospora indica [J/OL]. PLoS One, 2012, 7(4): e35502[2023-02-06]. . |
| 41 | PESKAN BERGHO T, GERSHENZON J, RAUSCH T. Sustained exposure to abscisic acid enhances the colonization potential of the mutualist fungus Piriformospora indica on Arabidopsis thaliana roots [J]. New Phytol., 2015, 208: 873-886. |
| 42 | 孙超. 印度梨形孢诱导小白菜抗病、促生、抗逆的作用及其机理的初步研究[D]. 杭州:浙江大学, 2010. |
| SUN C. Disease resistance, growth promotion and stress tolerance in Chinese cabbage conferred by Piriformospora indica and the preliminary study of mechanisms [D]. Hangzhou: Zhejiang University, 2010. | |
| 43 | WALLER F, MUKHERJEE K, DESHMUKH S D, et al.. Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species [J]. J. Plant Physiol., 2008, 165(1): 60-70. |
| 44 | JACOBS S, ZECHMANN B, MOLITOR A, et al.. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica [J]. Plant Physiol., 2011, 156(2): 726-740. |
| [1] | 马蓓, 公杰, 杜银柯, 甘雨薇, 程蓉, 朱波, 易丽霞, 马锦绣, 高世庆. 小麦花粉孔发育相关TaINP1基因鉴定及表达分析[J]. 中国农业科技导报, 2025, 27(4): 22-35. |
| [2] | 薛振宇, 张康康, 张元元, 闫强强, 姚立蓉, 张宏, 孟亚雄, 司二静, 李葆春, 马小乐, 王化俊, 汪军成. 优质抗旱小麦种质的筛选及功能基因检测[J]. 中国农业科技导报, 2025, 27(1): 35-49. |
| [3] | 孙宪印, 牟秋焕, 米勇, 吕广德, 亓晓蕾, 孙盈盈, 尹逊栋, 王瑞霞, 吴科, 钱兆国, 赵岩, 高明刚. 基于GT双标图对小麦新品系的分类评价[J]. 中国农业科技导报, 2024, 26(7): 14-24. |
| [4] | 鲍新跃, 陈红敏, 王伟伟, 唐益苗, 房兆峰, 马锦绣, 汪德州, 左静红, 姚占军. 小麦TaCOBL-5基因克隆及表达分析[J]. 中国农业科技导报, 2024, 26(6): 11-21. |
| [5] | 徐佳睿, 王逸茹, 赵绍赓, 李坤, 郑军. 玉米木质素合成途径基因ZmCCoAOMT1功能研究及转录组分析[J]. 中国农业科技导报, 2024, 26(5): 30-43. |
| [6] | 赵刚, 王淑英, 李尚中, 张建军, 党翼, 王磊, 李兴茂, 程万莉, 周刚, 倪胜利, 樊廷录. 黄土旱塬区近40年降水对冬小麦耗水和产量的影响[J]. 中国农业科技导报, 2024, 26(3): 164-173. |
| [7] | 张宏, 李卫国, 张晓东, 卢必慧, 张琤琤, 李伟, 马廷淮. 基于HJ-1星和GF-1号影像融合特征提取冬小麦种植面积[J]. 中国农业科技导报, 2024, 26(2): 109-119. |
| [8] | 张景云, 关峰, 石博, 万新建. 小麦根系分泌物对苦瓜幼苗生长及土壤生物学环境的影响[J]. 中国农业科技导报, 2024, 26(2): 181-190. |
| [9] | 李双, 王爱英, 焦浈, 池青, 孙昊, 焦涛. 盐胁迫下不同抗性小麦幼苗生理生化特性及转录组分析[J]. 中国农业科技导报, 2024, 26(2): 20-32. |
| [10] | 刘海霞, 张寅辉, 庄蕾, 郭梦娇, 赵李, 吴美娟, 侯健, 李甜, 刘红霞, 张学勇, 郝晨阳. 基于关联分析挖掘小麦SDS沉降值相关候选基因及KASP标记开发[J]. 中国农业科技导报, 2024, 26(12): 18-29. |
| [11] | 赵展, 王晓婷, 张黎凤, 赵津禾, 于玉红, 李军华, 吴占清. 西瓜对低氮胁迫响应的转录组分析[J]. 中国农业科技导报, 2024, 26(12): 30-38. |
| [12] | 胡墨明, 孟琰珺, 苗淑媛, 胡西宁, 许晴, 马红珍, 王鹏飞, 刘国芹, 康国章. 小麦淀粉合成关键基因TaAGPL1功能标记的开发[J]. 中国农业科技导报, 2024, 26(11): 43-55. |
| [13] | 田鹏, 宋洁, 李霞, 王裕智, 武棒棒. 不同粒色小麦籽粒中叶酸及衍生物含量分析[J]. 中国农业科技导报, 2024, 26(11): 56-65. |
| [14] | 岳洁茹, 秦志列, 侯起岭, 苑少华, 郝小聪, 杨吉芳, 白秀成, 赵昌平, 张风廷, 孙辉. BS型小麦光温敏雄性不育系柱头外露规律研究[J]. 中国农业科技导报, 2024, 26(10): 22-29. |
| [15] | 王韵弘, 苗琪, 李俊超, 王红叶, 张济世, 崔振岭. 田间管理措施对滨海盐渍地区中低产田生产力的影响[J]. 中国农业科技导报, 2024, 26(1): 163-172. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010802021197号
京公网安备11010802021197号